Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 1, Issue 2, April 2013, Pages 87-91
Original researchOpen Access
Antioxidant activity of some benzimidazole derivatives to definite tumor cell lines
- 1 Institute of Organic Chemistry, Bulgarian Academy of Science, 1113 Sofia, Bl.9, Bulgaria
- 2 University of Chemical Technology and Metallurgy, 8 Kliment Ohridski Blvd., 1756 Sofia, Bulgaria
- 3 Institute of Parasitology and Experimental Pathology Bulgarian Academy of Science, Bl.25, 1113 Sofia, Bulgaria
*Corresponding author: Wesselinova D, Institute of Parasitology and Experimental Pathology Bulgarian Academy of Science, Bl.25, 1113 Sofia, Bulgaria, Tel.: +359 9792388. E-mail: dianaw33@hotmail.com
Received 10 December 2012 Revised 13 February 2013 Accepted 20 February 2013 Published 27 February 2013
DOI: http://dx.doi.org/10.14312/2052-4994.2013-13
Copyright: © 2013 Goshev I, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
A group of bis(benzimidazol-2-yl) amines have been already evaluated for cytotoxicity in vitro to human colorectal cancer cell line HT-29, breast cancer cells MDA-MB-231 and normal spleen cells and two of them (B1 and B2) have been taken for the purposes of our present investigations. From the second group of compounds representing 1,3-disubstituted-2,3-dihydro-2-iminobenzimidazoles two substances (B3 and B4) have been chosen because of their most pronounced anti-proliferative effect to human colorectal cancer cell line HT-29, breast cancer cells MDA-MB-231 and normal spleen cells, using the in vitro proliferative MTS-test. It was important to estimate the cause for this suppressive activity of the compounds. We proposed that this could be due to their antioxidant capacity. The substances were examined for antioxidant activity against hydroxyl and peroxyl radicals, applying the HORAC and ORAC methods and showed considerable capacity. The scavenging capacity of B2 towards hydroxyl radicals is the highest, followed by B1. It was estimated that B2 has the greatest scavenger capacity of oxygen radicals, emitted by the examined cells followed in descending order by B1, B3 and B4. The observed differences can be considered as impact of their structure on the Me+2-helating activity and effective H-atom donation. A correlation was observed between the structure of the particular substance and the expressed antioxidant potential. The latter correlated also with the effect on the tested tumor cell lines. This result means that tumor cells are accompanied by a measurable emission of ROS which might be regulated by a proper application of antioxidants.
Keywords: aminobenzimidazoles; HORAC; ORAC; antioxidant activity; tumor cells
IntroductionTop
The massive production of ROS in an inflammatory environment (characterized as oxidative burst) plays a key role in defense against environmental pathogens. In an inflammatory environment, activated neutrophils or macrophages produce large quantities of superoxide radical and other ROS via the phagocytic isoform of NAD(P)H oxidase. Various types of nonphagocytic cells involving fibroblasts, vascular smooth muscle cells, cardiac myocytes, and endothelial cells are known to produce ROS by NAD(P)H oxidase to regulate intracellular signaling cascades. The immune response is redox regulated process where interleukin-2 production can be induced by relevant concentrations of superoxide radical and hydrogen peroxide and the programmed cell death (apoptosis) is needed both for proper development and to destroy cells that represent a threat to the integrity of the organism. Another question remains open – which molecules are targets of the stress induced in cells by ROS. ROS certainly oxidize membrane lipids, but the amount of ROS emitted after T cell activation is too small to mediate lipid peroxidation and membrane destruction [1]. It is known that metal-induced generation of ROS results in an attack not only on DNA, but also on other cellular components involving polyunsaturated fatty acid residues of phospholipids, which are extremely sensitive to oxidation. Mechanisms involved in the oxidation of proteins by ROS were elucidated by studies in which amino acids, simple peptides and proteins were exposed to ionizing radiation under conditions where hydroxyl radicals or a mixture of hydroxyl/ superoxide radicals are formed [2]. Most cell types have been shown to elicit a small oxidative burst generating low concentrations of ROS when they are stimulated by cytokines, growth factors and hormones. The abnormal behavior of neoplastic cells can often be traced to an alteration in cell signalling mechanisms, such as receptor or cytoplasmic tyrosine kinases, altered levels of specific growth factors. It has been clearly demonstrated that ROS interfere with the expression of a number of genes and signal transduction pathways and are thus resources in the process of carcinogenesis [3]. The exposure to free radicals from a variety of sources is the reason for development of series of defense mechanisms in organisms against the free radical-induced oxidative stress. A more reducing conditions (maintained by elevated levels of glutathione and thioredoxin) of the cell stimulates proliferation and a slight shift towards a mildly oxidizing environment initiates cell differentiation. A further shift towards more oxidizing conditions in the cell leads to apoptosis and necrosis.
Among the naturally occurring antioxidants as flavonoids quercetin, kaempferol, delphinidin, vitamin E and as well as the synthetic compounds, which are studied for their antioxidant activity, the bezimidazole derivatives occupied essential position [4, 5]. The antioxidative properties of many new benzimidazole derivatives were determined in vitro on the rat liver microsomal NADPH-dependent lipid peroxidation (LP) level, the scavenging of superoxide anion and the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), so 5-nitro-2-(phenoxymethyl)benzimidazole invokes strongly inhibition (91%) to lipid peroxidation at 10-3 M concentration [6]. 5-Ethoxy-2-ethylthiobenzimidazole hydrochloride known as ethomerzol prevents lipid peroxidation activation and antioxidant system suppression in brain and liver of albino rats at acute hypoxia at concentration25 mg/kg, intraperitaneally, 30 min before hypoxia [7]. Compounds 2-(3-chlorostyryl)-1H-benzimidazole and 2-(4-chlorostyryl)-1H-benzimidazole are found to be moderately active, having IC50 values 99.5 μM/L and 95.5 μM/L respectively as compared to 89.4 μM/L of standard ascorbic acid by nitric oxide scavenging method. 2-Styryl-1H-benzimidazole, 2-(2-chlorostyryl)-1H-benzimidazole and 2-(2-(1H-benzimidazol-2-yl)vinyl)phenol have their IC50 values as 73.2 μM/L, 61.5 μM/L and 61.1 μM/L respectively and are more potent than standard antioxidant ascorbic acid [8] Thiazine derivatives of benzimidazole were found to show potent antioxidant activity [9] and benzimidazoles containing pyridopyrimidine ring as substituent showed the same effect with IC50 value of 10 μg/mL compared to the used commercial ascorbinic acid [10]. A benzimidazole compound containing both tetrahydronaphthalene and 4-phenylpiperazine fragments in molecule displayed scavenging effect at 10-3 M concentration (98%) on superoxide anion radical, a result which is better than that achieved with 30 IU of SOD (76%) [11].
Having in view the above mentioned facts, it is obvious that the study of the antioxidant properties of other benzimidazoles is of pharmacological interest. In this paper we report the examination of antioxidant activity of some benzimidazole derivatives, which were found to display a cytotoxic effect on tumor cell lines (HT-29, MDA-MB-231) respectively proliferating activity against normal spleеn cells.
The decision to examine the antioxidant activity of the substances toxic toward tumor cells came from the available data that all living cells (including tumor cells) emit ROS. In the case that cancer cells (like all other living cells) [3] emit ROS (and maybe in greater extent!) and the examined compounds scavenge them, the tumor cells should be suppressed and/or even die! Our preliminary tests (HORAC & ORAC) have shown that these radicals cannot be detected on the tumor cells, maybe because of their low amount and/or very short life. The vice versa way to prove the presence of such radicals was to incubate the tumor cells with antioxidants and to estimate the cellular suppressive or proliferative response.
Materials and methodsTop
The bis(benzimidazol-2-yl)amines (B1 and B2) were obtained by substituition of the sulfuric group in the benzimidazol-2-sulfonic acid through appropriated benzimidazole-2-amines and were discussed and reported by Mavrova et al. [12]. The derivatives of 2,3-dihydro-2-imino-1H-benzimidazoles B3 and B4 were synthesized by nucleophilic substitution of 2-amino-benzimidazoles under solid-liquid phase transfer catalysis conditions in dry acetonitrile as well as in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and detailed description is given in the paper of Mavrova et al [13].
The chemical structures of the compounds were established by elemental analyses, IR-, 1H NMR and 13C NMR spectra and the results are presented in the Supplementary Material part. The elemental analyses indicated by the symbols of the elements were within ± 0.4% of theoretical values.
Cytotoxicity
All compounds were evaluated in vitro earlier [12, 13] for their cytotoxicity
to human colorectal cancer cell line HT-29, breast cancer cells MDA-MB-231 (American
Type Culture Collection, Rockville, MD, USA) and normal spleen cells by using the
MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrasolium
inner salt) test. The cell proliferation MTS-assay is based on the fact that the
MTS tetrazolium compound is bio-reduced by cells into a colored formazan product
that is soluble in the tissue culture medium. This conversion is presumably accomplished
by NADPH or NADH, produced by dehydrogenase in metabolically active cells. The greater
release amount of formazan indicates to a higher vitality of the cells (proliferation).
A low vitality demonstrates a cytotoxic influence of all experimental substances
(to all cellular kinds).
ROS-measurements
Preparation of the samples
Approximately 2 mg of each sample were dissolved in 2 mL acetone:distilled water:
conc. acetic acid =70:29.5:0.5 at room temperature for 1 h. This solvent system
is widely applied for enhanced extraction of phenolic substances from plant materials,
foods etc. [14]. According to a preliminary experience, a more appropriate solvent
of the tested substances is DMSO. It is a free radical scavenger, especially in
the case of hydroxyl radical [14]. Samples B1, B2 and B4 do not dissolve entirely
and were sonicated additionally (20 kHz, 30 W, 1 min at room temperature). The suspensions
obtained were applied for determination of the antioxidant capacity immediately.
Determination of the antioxidant capacity
HORAC – Hydroxyl Radical Averting Capacity
The method is based on in situ
generation of hydroxyl radicals (OH•) by catalytic decomposition
of hydrogen peroxide by divalent metal salts (Co2+) at 37°C and pH
7.4 (75 mM sodium phosphate buffer) [15]. The reaction is performed in 10 mm light
path quartz fluorescence cell on Perkin Elmer LS5 fluorometer equipped with thermo
stated cell holder. Fluorescein-disodium salt was used for monitoring of the free
radical generation and their scavenging by the tested substances (λex=493 nm;
λem=518 nm, observation period 30 min). Gallic acid (GA) was used as a standard.
The results are expressed micromole GA equivalents/mol substance.
Oxygen radical absorbance capacity
The experiment is carried out on the same equipment, in the same buffer and applies
also fluorescein-disodium salt as a probe [16, 17]. The generation of oxygen radical
(peroxyl radical, RO2•) is achieved by thermal decomposition
of AAPH (2,2’-azobis-2-methyl-propanimidamide dihydrochloride, purchased from Cayman
Chemical Co.) at 37°C and pH 7.4 in the above mentioned phosphate buffer. TROLOX®
(6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, a water-soluble derivative
of vitamin E, purchased from Sigma-Aldrich) was used as standard. The results are
expressed as micromole TROLOX equivalents/mol substance.
Treatment of experimental data
For both standard substance and running sample sigmoid fluorescence decay curves
were obtained. The antioxidant potential of the sample is evaluated by comparison
of its net area (NA) with that of the standard. For its determination is used the
so called “Area Under Curve” – AUC, calculated for both sample and standard
![]()
-by subtracting the AUC of the blank (buffer) we obtain the NA-values for both sample
and standard
The final expression which describes the antioxidant potential of the sample is:
![]()
Where the effective concentrations (in the photometric cell) of sample and standard
are used. The final result is in mol Standard equivalents/mol sample.
ResultsTop
The synthesis of compounds B1-B4 was performed as illustrated in Figure 1:
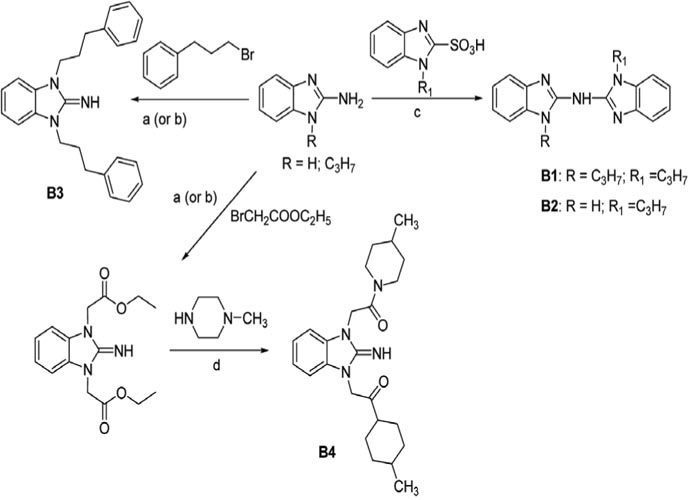
Figure 1 Scheme of the of 2-aminobenzimidazole derivatives B1-B4 synthesis. a) DBU acetonitrile, halogen derivative, 20°C; (b) acetonitrile, TBAB, dry K2CO3; 20°C; (c) 180°C for 30 min; (d) ethanol, 1-methylpiperazine, refluxing, 4 h.
Relative cell viability of the tested by MTS-test compounds, expressed as a percentage of the untreated control (100% viability), was calculated for each concentration [12, 13]. All data points represent an average of three independent assays and the obtained results were plotted and IC50 and EC50 were calculated. The data are given in Table 1 and Table 2.
| Comp | IC50± SE(μM) | EC50± SE(μM) | ||||
| HT-29 | MDA-MB-231 | Normal spleen cells | HT-29 | MDA-MB-231 | Normal spleen cells | |
| B1 | 1.92 ± 0.08 | 0.006 ± 0.27 | NDa | ND | ND | 0.5.10-4 ± 0.6 |
| B2 | 0.91 ± 0.11 | 0.135 ± 0.06 | 0.11 ± 0.62 | ND | ND | ND |
Abbreviations: anot detected
| Comp | IC50±SE(nM) | EC50±SE(nM) | ||||
| HT-29 | MDA-MB-231 | Normal spleen cells | HT-29 | MDA-MB-231 | Normal spleen cells | |
| B3 | 9.26 ± 0.11a | ND | ND | 1.338 ± 0.22 | 14.13± 0.59 | |
| B4 | 0.013 ± 0.81 | ND | 0.38 ± 0.04 | ND | 1.59 ± 0.12 | |
aStatistical significant differences in the level of cells in both control and experimental groups were determined (p ≤ 0.05)
The data for the expressed antioxidant capacity towards hydroxyl radical (HORAC) and peroxyl radical (ORAC) (made separately from the MTS-experiment!) are summarized in Table 3 and the graphical presentation of the data obtained is shown in Figure 2. It is evident that all tested samples show measurable antioxidant capacity which is definitively influenced by their structural peculiarities. According to the hydroxyl radical scavenging capacity the investigated substances can be ordered as follows: B2>B1>>B3>B4 (B3~B4).
| Sample index | Molecular mass, Da | HORAC, mM GAE/g | HORAC, M GAE/M | ORAC, mM TrE/g | ORAC, M TrE/M |
| B1 | 333.43 | 24.43 | 8.15 | 2.62 | 0.87 |
| B2 | 291.35 | 61.10 | 17.80 | 5.10 | 1.48 |
| B3 | 369.50 | 4.96 | 1.83 | 5.27 | 1.95 |
| B4 | 413.52 | 3.62 | 1.50 | 3.09 | 1.28 |
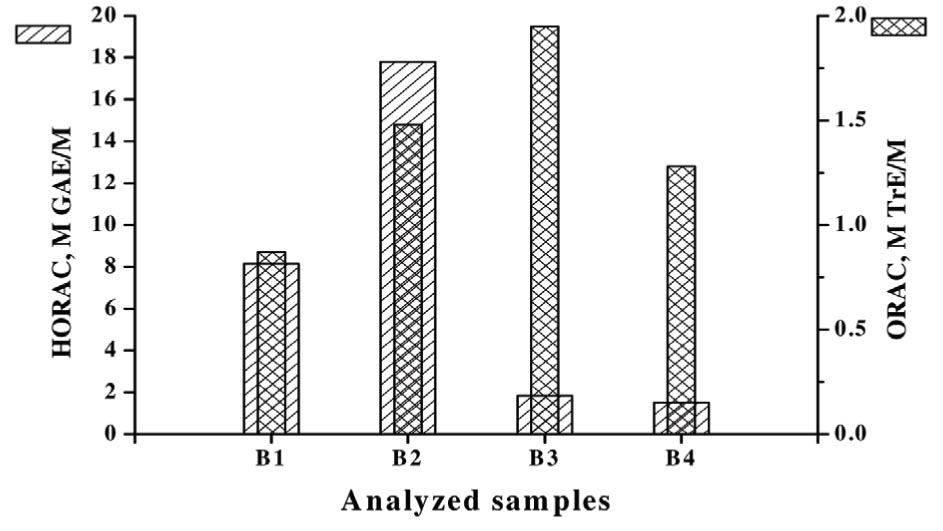
Figure 2 Graphical presentation of the data obtained about the ROS-scavenger activity of the examined substances.
DiscussionTop
As it can be seen from Table 1 compound B1 showed relative high cytotoxicity against HT-29 and MDA-MB-231 cells (IC50-0.006 μM ) but in the same time that compound revealed proliferative effect toward normal spleen cells (EC50-0.5.10-4 μM). Respect to the results shown by the other 2-aminobezimidazole derivatives (Table 2) it may be pointed that B3 exhibited cytotoxicity against HT-29, but is not toxic against MDA-MB-231 and normal spleen cells, while compound B4 showed the highest toxicity against HT-29 (IC50-0.013 nM). If the results, obtained for compound B1 are taken in consideration it should be noted that 1-propyl-N-(1-propyl-1H-benzimidazol-2-yl)-1H-benzimidazol-2-amine (B1) revealed proliferative effect against normal spleen cells at lower concentration in comparison to the concentration at which the compound exerted cytotoxic effect to HT-29 cells. Besides it manifested cytotoxic effect to MDA-MB-231 cells and proliferative activity to normal spleen cells at low concentration.
In the same time its antioxidant capacity towards hydroxyl radicals however is definitely low (Figure 2). The scavenging capacity of B2 towards these radicals is the highest, followed by B1. The hydroxyl radical emission of the cells (HORAC) is in any case stronger in comparison to this of the oxygen emission. It was estimated that B2 has the greatest scavenger capacity of oxygen radicals followed in descending order by B1, B3 and B4. B3 has a pronounced toxic effect to the MDA-MB-231 cells, whereas it has no effect to other cell species. We could assume that the antioxidant activity of B2 correlates well with its cytotoxicity, because it can scavenge the oxygen radicals from the tumor cells better, than from the normal cells. Our suggestions were in the direction whether the free radical scavenging ability of the tested substances may stimulate their suppressor activity. And not only this, we expect that tumor cells emit higher amounts of the mentioned radicals and depending on their structure, the examined substances would serve as effective scavengers and would suppress the tumor cells’ development, but in different manner and extent.
On the base of the promising screening results it might be concluded that the selectivity of compound B2 could be essential for anticancer drug discovery.
According to the observed hydroxyl radical scavenging capacity (i.e. effective helating of Me2+), the investigated substances can be ordered as follows: B2>B1>>B3>B4 (B3~B4). Sample B2 might be considered as a multi-center complex forming agent (multidentate ligand). The additional alkylation in substance B1 significantly limits its complex formation ability resulting in approximately two times lower HORAC-value. Substances B3 and B4 are not typical helators and have bulky substituents, which additionally limit the access to the nitrogen atom. The carbonyl groups in B2 may form internal adducts with the imino-group thus preventing its interaction with metal ions. In other words, the position and environment of the available nitrogen atoms in B3 and B4 are not appropriate for effective complex formation with metal ions, and these results in quite low HORAC-values.
The observed ORAC (oxygen radical absorbance capacity) values of the tested substances raise the question for their HA-donation capability. We have two pairs either substituted to a different extent – B2 vs B1 or differing in symmetrically situated bulky substituents – B3 vs B4. Interesting fact is that these structural peculiarities affect almost equally the observed ORAC-values, e.g. ∆B2/B1 =1.48–0.87 =0.61 M TrE/M and ∆B3/B4 =1.95–1.28 =0.67 M TrE/M. In the case B2 vs B1 there is a change from 2 HA-donation sites to a single one. In the case B3 vs B4 the substituents differ in their structure and a possible HA-donation site is the imino-group. In B3 it may still be effective, but in B4 the spatially close carbonyl groups may form internal adduct(s) by means of H-bond(s) thus reducing the HA-donation capability.
ConclusionTop
In conclusion we have to note that substance B2 is essential in prevention of hydroxyl radical generation. Substance B3 is essential in HA-donation ability thus limiting the effect of peroxyl radicals, but the capacity of the other substances is of a comparable magnitude, even exceeding the capacity of TROLOX, a water-soluble analog of the well-known lipophilic physiological antioxidant Vitamin E. In this sense, although a little bit speculatively, the peroxyl radical scavenging capacity seems to be a dominating feature of the tested set of substances.
Conflict of interest
All the authors declare that they have no conflict of interest.
ReferencesTop
[1] Hildeman DA, Mitchell T, Kappler J, Marrack P (2003) T cell apoptosis and reactive oxygen species. J Clin Invest 111: 575–581. Article Pubmed
[2] Stadtman ER (2004) Role of oxidant species in aging. Curr Med Chem 11: 1105–1112. Article Pubmed
[3] Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84. Article Pubmed
[4] Gurer-Orhan H, Orhan H, Suzen S, Püsküllü MO, Buyukbingol E (2006) Synthesis and evaluation of in vitro antioxidant capacities of some benzimidazole derivatives. J Enzyme Inhib Med Chem 21: 241–247. Article Pubmed
[5] Rajasekaran S, Rao G, Chatterjee A (2012) Synthesis, Anti-Inflammatory and Anti-oxidant activity of some substituted Benzimidazole Derivatives. Int J Drug Dev & Res 4: 303–309. Article
[6] Temiz-Arpaci O, Coban T, Tekiner-Gulbas B, Can-Eke B, Yildiz I, et al. (2006) A study on the antioxidant activities of some new benzazole derivatives. Acta Biol Hung 57:201–209. Article Pubmed
[7] Mironova OP, Zarubina IV, Shabanov PD (2003) Ethomerzol as an antioxidant. Biomed Khim 49:434–442. Article Pubmed
[8] Chhajed S. S, Upasani C. D (2011) Synthesis and Antioxidant Activity of Some Novel 2-Substituted Analogues of Benzimidazoles. J Pharm Res 4:340–343. Article
[9] Ramjith US, Cherian A, Jacob CM (2012) Microwave Assisted Synthesis of 2-substituted Benzimidazole Thiazine Derivatives for Antioxidant Activity. Inventi Rapid: Med Chem. Article
[10] Maheswaran N, Saleshiera MF, Mahalshmia K, Sureshkannan V, Parthiban N, Reddy KA (2012) Synthesis and Characterisation of 7-(1H-Benzimidazol-2-yl)-5-(Substituted Phenyl) Pyrido [2, 3-D] Pyrimidin-4-Amine for their Biological Activity. Int J Chem Sci 10: 43–51. Article
[11] Ateş-Alagöz Z, Kuş C, Coban T (2005) Synthesis and antioxidant properties of novel benzimidazoles containing substituted indole or 1,1,4,4-tetramethyl-1,2,3,4-tetrahydro-naphthalene fragments. J Enzyme Inhib Med Chem 20:325–331. Article Pubmed
[12] Mavrova AT, Wesselinova D, Tsenov JA, Denkova P (2012) Cytotoxic Effects of some N-Substituted-2-Amino-1H-Benzimidazoles. J Bioequiv Availab 4: 52–55. Article
[13] Mavrova ATs, Wesselinova D, Vassilev N, Tsenov JA (2011) Synthesis, characterization and cytotoxicity of some novel 1,3-disubstituted-2,3-dihydro-2-iminobenzimidazoles. Eur J Med Chem 46: 3362–3367. Article Pubmed
[14] Singleton VL, Rossi JA (1965) Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Аm J Еnоl Vitic 16: 144–158. Article
[15] Total ROS Detection Kit for fluorescence microscopy and flow cytometry. Instruction Manual Cat. No. ENZ-51011, Rev. 2.0.2 November 2010. Article
[16] Ou B, Hampsch-Woodill M, Flanagan J, Deemer EK, Prior RL, et al. (2002) Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J Agric Food Chem 50: 2772–2777. Article Pubmed
[17] Yilmaz Y, Toledo RT (2004) Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid J Agric Food Chem 52: 255–260. Article Pubmed
[18] Wada L, Ou B (2002) Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem 50: 3495–3500. Article Pubmed



