Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 1, Issue 6, August 2013, Pages 153-162
Original researchOpen Access
Pharmacological and safety evaluation of CIGB-300, a casein kinase 2 inhibitor peptide, administered intralesionally to patients with cervical cancer stage IB2/II
-
Soriano-García JL1,
López-Díaz A2,
Solares-Asteasuainzarra M3,
Baladrón-Castrillo I4,
Batista-Albuerne N1,
García-García I4,
González-Méndez L4,
Perera-Negrín Y4,
Valenzuela-Silva CM4,
Pedro AP2,
Quevedo-Sotolongo LS5,
Hernández-González I6,
Silveira-Pablos JM7,
Chong-López A8,
Alonso DF9,
Gómez RE10,
Renault JY11,
Perrin P11,
Sigman H12,
Gold S12,
Perea-Rodríguez SE4,
Acevedo-Castro BE4,
Herrera-Martínez L4,
López-Saura PA1,*
 ,
and CERVIFARM-300 Study Group.
,
and CERVIFARM-300 Study Group.
- 1 Oncology Department, Hermanos Ameijeiras Hospital, Havana, Cuba
- 2 Nuclear Medicine Department, Hermanos Ameijeiras Hospital, Havana, Cuba
- 3 National Reference Center for Cervical Cancer Research, Gyneco-obstetric Hospital Ramón González Coro, Havana, Cuba
- 4 CIGB-300 Research and Development Group, Center for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba
- 5 Imaging Department, Central Clinic Cira García, Havana, Cuba
- 6 Isotope Center (CENTIS), Havana, Cuba
- 7 Oncology Department, National Institute for Oncology and Radiobiology, Havana, Cuba
- 8 Pathology Department, Hermanos Ameijeiras Hospital, Havana, Cuba
- 9 Molecular Oncology Laboratory, National University of Quilmes, Buenos Aires, Argentina
- 10 ELEA Laboratories, Buenos Aires, Argentina
- 11 ChemoFrance Division, ChemoFrance, Paris, France
- 12 Chemo Group, Buenos Aires, Argentina
*Corresponding author: García-García I, Clinical Investigation Department, Center for Genetic Engineering and Biotechnology, Ave 134 b/23 and 25, Cubanacán, Playa, Havana, P.O. Box 6332, Cuba, Tel.: +53 (7) 2087379/ 2087465; Fax: +53 (7) 2736008. E-mail: idrian.garcia@cigb.edu.cu
Received 13 April 2013 Revised 1 July 2013 Accepted 9 July 2013 Published 17 July 2013
DOI: http://dx.doi.org/10.14312/2052-4994.2013-25
Copyright: © 2013 Soriano-García JL, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
CIGB-300 is a pro-apoptotic casein kinase 2 inhibitor peptide with potential anticancer action. An open-label and dose scaling Phase I trial was carried out to investigate the peptide tumor uptake, pharmacokinetics, toxicity, and levels of a CIGB-300 response biomarker in patients with cervical cancer stage IB2/II. Fourteen patients were included; six of them received 35 mg, 6 received 70 mg and the two remaining patients received 245 mg of CIGB-300 prior chemoradiotherapy. CIGB-300 was applied by intratumor injections during 5-consecutive days. For pharmacokinetic and biodistribution studies, the peptide was radiolabeled with 99mTc in the first administration and whole body gammagraphy and plasma testing were done during 48 h. Data showed that the maximum tolerated dose was 70 mg for CIGB-300 in this clinical setting. Furthermore, an allergic-like syndrome was identified as the dose limiting toxicity, which was well-correlated with plasmatic histamine levels. Importantly, the mean tumor uptake was 14.9 mg and 10.4 mg for CIGB-300 doses of 35 and 70 mg, respectively. Also, the kidneys were the main target organ for drug elimination. Finally, treatment with CIGB-300 significantly reduced the B23/nucleophosmin levels in tumor specimens. CIGB-300 meets potentialities to be tested in future trials in a neoadjuvant setting prior to chemoradiotherapy in cervical cancer.
Keywords: CIGB-300; cervical cancer stage; tumor uptake; casein kinase; pharmacokinetics; histamine; B23/nucleophosmin
IntroductionTop
Every year more than half million of new cases of cervical cancer patients are diagnosed worldwide, 80% of them in developing countries. Around 260 000 women die because of this illness, second cause of death after breast cancer [1]. Current standard therapy for inoperable cervical cancer stage IB2/II is based on regimens that include concomitant chemoradiotherapy [2]. These treatments remain to be suboptimal [3] with some inconveniences either at short or long term [4, 5]. Such data along with knowledge about the natural history of the disease suggest that stage IB2/II cervical cancer still needs evaluation of novel biological therapies to be evaluated either as neoadjuvant or adjuvant treatment to chemoradiotherapy, in order to prevent or reduce tumor progression toward later stages. Therefore, kinase inhibitors are a newcomer class of targeted therapy which has successfully entered into clinical research in cancer at present [6-8].
CIGB-300 is a novel pro-apoptotic peptide that impairs the CK2-mediated phosphorylation of the Human Papillomavirus E7 oncoprotein and the multifunctional oncoprotein B23/nucleophosmin (B23/NPM) [9-12]. Furthermore, CIGB-300 elicits antitumor activity in different animal models when administrated by different routes [13]. As an investigational drug, CIGB-300 was previously tested in a First-in-Human trial where patients with high grade squamous intraepithelial lesion (HSIL) or epidermoid microinvasive cervical cancer received intralesional injections of this peptide-based drug. These previous findings evidenced that CIGB-300 was safe and well tolerated in that clinical setting. Transient, dose-dependent, systemic "allergic-like" reactions were the main safety feature, and some clinical activity was evidenced by colposcopy, histology and anti-HPV response [14]. That was the first clinical trial in which a drug was used to target the CK2 phosphoaceptor domain providing an early proof-of-principle of a possible clinical benefit.
This study aimed to evaluate the tumor uptake, biodistribution and pharmacokinetics of CIGB-300 after local intratumoral injections to patients with cervical cancer stage IB2/III. Furthermore, the maximum tolerated dose (MTD), dose limiting toxicity (DLT) and a CIGB-300 response biomarker were explored in this clinical study.
Patients and methodsTop
Eligibility criteria
Patients histologically diagnosed with stage IB2/II cervical cancer were recruited in nine gyneco-obstetric departments throughout Cuba. They were then evaluated at the National Reference Center for Cervical Cancer at the "Ramón González Coro" Hospital, where the eligibility criteria were verified. Product administrations and evaluations took place, as inpatients, at the “Hermanos Ameijeiras” Hospital.
Patients were included if they were 18 to 75 years-old, had clinical, imaging and histological diagnosis of stage IB2/II cervical epidermoid carcinoma (FIGO Classification Stage) [15], a WHO general health index from 0 to 2, more than 1 year of life expectation and gave their written, informed consent to participate. Exclusion criteria were chemotherapy or surgical, ablative, radiant, or immunomodulator treatment in the previous 30 days, psychiatric disease, pregnancy, breastfeeding, uncontrolled chronic diseases such as asthma, epilepsy, diabetes or hypertension, autoimmune disease, coagulation dysfunction, acute systemic or genital tract infections, body mass index below 19 or above 30, current administration of immunomodulating drugs, tumor extensive necrosis that could limit the application of the product and participation in another clinical trial.
The study was conducted to Good Clinical Practice in accordance with the Declaration of Helsinki and its amendments. The protocol was approved by the Ethic Committees of the participating institutions, and by the Cuban Regulatory Authority.
Study design and treatment plan
An open, dose scaling, sequential trial was carried out. Patients were sequentially included to 4 dose groups (35, 70, 245, and 490 mg) of CIGB-300, according to the previous study [14]. It was expected to include six patients in each group but initially blocks of three per dose level were enrolled to assure pharmacological evaluation. Afterwards, if the maximum dose cannot be reached or if limiting toxicity impeded continuity with higher doses, the inclusion would be restarted in those groups with safe doses up to complete the six patients per group.
The sample size was inferred starting from a prefixed maximum limit of toxicity taking into account prior experience. That limit was 30% of patients with severe adverse events in each group, with a 95% confidence interval. This method for N calculation is considered specific to pilot studies with transitional therapies even when no adverse events (AE) are registered [16].
Patients were hospitalized and treated once daily during 5 days. CIGB-300 was injected into the tumor, approximately 2 cm deep, during 3-5 min, on one site as close as possible to the tumor center. Injection of necrotic area with craters or in the cervical channel hole was avoided. Tumor uptake, whole body distribution and drug levels in blood were measured after the first drug administration with 99mTechnetium (Tc)-labeled CIGB-300 for all the patients.
CIGB-300 was synthesized and supplied by the Peptide Synthesis Department, CIGB, Havana, in 35 mg vials, as a lyophilized powder. The content of 1, 2 or 7 vials was reconstituted in water for injection to a final volume of 2 ml in all of the cases. Other interventions were indicated only for the management of adverse events, according to established clinical practices.
Conventional radio-chemotherapy was started 25-35 days after CIGB-300 treatment according to specific international guidelines for this pathology followed by the oncology department of the “Hermanos Ameijeiras” Hospital. The regimen received by all the patients consisted in cisplatin 40 mg/m2, once per week, during 6 weeks, concomitant to 5040 centigrays (cGy) of external beam radiotherapy given in 28 fractions. This was followed by 2600 cGy of intracavitary radiotherapy given in 4 high dose fractions (650 cGy/day) over 2 weeks.
Safety evaluation
The pretreatment evaluation included a detailed history and physical examination. In addition, electrocardiogram, hematological counts, blood chemistry, coagulation and cervix microbiological studies were performed. Since one of the main purposes of the trial was to evaluate safety, local and systemic AE, including routine laboratory parameters, were carefully screened up. Systemic toxicity was evaluated during 24 h after each CIGB-300 administration, including cardiovascular monitoring during the injections and vital signs measurements (temperature, heart beats, respirations/ min, blood pressure). Patients were followed for safety during and at 3, 6, 9, and 12 months after chemoradiotherapy.
The medical terminology for AE and their severity classification (in 5 grades) was applied according to the Cancer Therapy Evaluation Program, Common Terminology Criteria [17]. The causal relationship was classified as very probable (definitive), probable, possible or remote (doubtful) [18].The MTD was the highest dose level at which ≤30% of patients experienced DLT (grade 3 AE).
Plasma histamine levels were measured in order to be correlated both with both the severity of the expected allergic reactions and the CIGB-300 blood concentration. A commercial EIA kit (Labor Diagnostika Nord, Nordhorn, Germany) was used to quantify histamine before, 15 min and 24 h after the first application.
Biodistribution analysis
The peptide was radiolabeled with 30-50 mCi of 99mTc by a direct method using dimercaptosuccinic acid (DMSA) as coligand [13]. Whole body imaging studies were performed at 10 min and 1, 2, 4, 8, 12, 16, 24, 36 and 48 h after injecting the radiolabeled peptide and using a gamma camera (Phillips) with a high energy collimator for 99mTc. Tumor static views were also taken at 30 min and 4:30 and 24:30 h. The calculations of the percent of uptake radioactivity in each source organ and tumor were based on the Medical Internal Radiation Dose (MIRD) methodology for quantitative radiopharmaceutical biodistribution data acquisition [19]. Tumor mass was estimated from morphometric data obtained from initial Magnetic Resonance Imaging (MRI). Biological (elimination) half-life was adjusted by means of the Microcal Origin program, version 6.0. All of these data were introduced to an excel spreadsheet for parameters prosecution and calculation of the CIGB-300 up taken in tumor and organs. Monoexponential and biexponential functions were fitted for calculation of the area under the curve by analytical integration from 0 to the last experimental time.
Pharmacokinetic study
Blood samples were drawn by venipuncture immediately, 5, 15, 30 min and 1, 2, 4, 8, 12, 16, 24, 36 and 48 h after the administration of the radiolabeled peptide (first dose). Urine was also collected during this sampling period.
The radioactivity in whole blood, serum and urine samples was measured in a gamma-well counter (CAPINTEC, CAPRAC-R). To correct for radioactive decay, injection standards were counted simultaneously. Radioactivity of blood samples was expressed as the percentage of injected dose per liter (%ID/L). Afterwards this percentage was transformed to the equivalent mass unit (µg/ml).
Drug disposition data analysis was performed individually by a non-compartmental method with a combined linear/log - linear trapezoidal rule approach. All the pharmacokinetic parameters were determined by using the WinNonlin professional software (version 5.1, Pharsight Inc., 2005, NC, USA).
Immunohistochemistry of B23/nucleophosmin and CK2β in tumor biopsies
Expression of B23/NPM and CK2β in tumors was studied by immunohistochemistry in paraffin-embedded tumor biopsies taken before and after CIGB-300 treatment using a commercial immunostaining kit (Histostain-SP, Zymed Laboratories, USA). Briefly, 4 µm tumor sections were deparaffined in xylene and rehydrated in a graded series of ethanol. Subsequently, slides were boiled in 10 mm during 20 min in citrate buffer for antigen retrieval. Primary antibodies were incubated as recommended by manufacturers; anti-B23/nucleophosmin (Zymed, 32-5200) and anti-CK2β (Santa Cruz, sc-20710). After several washes, the incubation with the respective secondary biotinylated antibodies was done during 30 min at room temperature. Slides were washed again and incubated with the streptadivine-peroxidase conjugate in the same conditions. Peroxidase stain and further contra stained with hematoxylin was also performed as recommended by manufacturer.
Statistical analysis
Data were double entered and validated on MS InfoPath and then imported into SPSS for Windows version 15.0 for further analysis. Continuous variables were expressed as mean ± standard deviation (SD) or median ± quartile range (QR) and minimum and maximum values (range). With these variables analyses of normality (Shapiro Wilk’s test) and homogeneity of variance (Levene’s test) were carried out. Categorical variables were given as absolute values and percentages. The homogeneity between the dose groups was analyzed by the estimation of the Bayes’ factor toward the hypothesis of equality and their posterior probability. Biodistribution and pharmacokinetic parameters as well as those variables related with the therapeutic effect (imaging, biological) were tested by the estimation of the 95% confidence intervals for the differences between groups as well as the calculation of the probability that the difference was > 0. Tolerability was treated through the estimation of severe toxicity in each group conditioned by the number of patients included sequentially in each group. For all the groups the probability to surpass maximum level of toxicity (30%) was estimated. Bayes’ factor toward the hypothesis of dependency between the groups respect to the registered AE was also estimated together with the probability of independency. Spearman rank correlation analyses between histamine levels and CIGB-300 concentration and between histamine and severity of the allergic reaction were also done.
ResultsTop
Patient characteristics and dosing history
Thirty-four patients were recruited from March to October 2008; fourteen of them fulfilled the selection criteria. The main causes of exclusion were the presence of endophytic tumor (6 cases) and other cervical cancer stages (4 cases). Patient follow-up continued up to one year after completion of therapy.
Baseline characteristics of the enrolled patients are shown in Table 1. The hypothesis of homogeneity between the groups was accepted. Most of the patients were white. Mean age was 43 years, ranging from 31 to 67 years. Weight, height and body mass index were similar among groups. No gynecological or general pathological antecedent stood out particularly (data not shown).
| Variable | Group I (35 mg)N = 6 | Group II (70 mg) N = 6 | Group III (245 mg) N = 2 | BF (H0Prob) |
| Skin color | ||||
| White | 4 (66.7%) | 5 (83.3%) | 1 (50.0%) | 1.50 (0.40) |
| Non-white | 2 (33.3%) | 1 (16.7%) | 1 (50.0%) | |
| Age (years) | 42 ± 8 (33 - 52) | 47 ± 12 (35 - 67) | 39 ± 11 (31 - 46) | |
| Weight (Kg) | 72 ± 10 (57 - 86) | 69 ± 14 (51 - 86) | 69 ± 10 (62 - 76) | |
| Height (cm) | 161 ± 7 | 161 ± 6 | 170 ± 13 | |
| (151 - 169) | (154 - 167) | (161 - 179) | ||
| Body Mass Index (Kg/m2) | 27.7 ± 2.0 | 26.7 ± 5.6 | 23.8 ± 0.1 | |
| (24.8 - 30.1) | (21.2 - 35.2) | (23.7 - 23.9) | ||
Data are reported as number of patients (%) or mean ± standard deviation (range);
BF: Bayes’ factor toward the hypothesis of dependency;
(H0 Prob): Probability of independency
This trial was developed through a sequential scaling-dose design according to safety data. Initially, three patients were included in the smallest dose (35 mg) without the presence of severe adverse reactions. Later on, other three patients received the following dose (70 mg), likewise without severe toxicity. Therefore, the inclusion in the 245 mg group was initiated but grade 3 events (allergic reactions) appeared after the first dose in the first two enrolled patients leading to definitive interruption of inclusion in this group and in the next one (490 mg). In a second period, the inclusion restarted with three patients in the 35 mg group where no severe events occurred. After that, during the administration of 70 mg, only patients #12 and #14 presented grade 3 allergic reactions, considering this dose as the MTD (Figure 1). In conclusion, 6 patients were included in both 35 and 70 mg groups whereas other 2 received 245 mg.
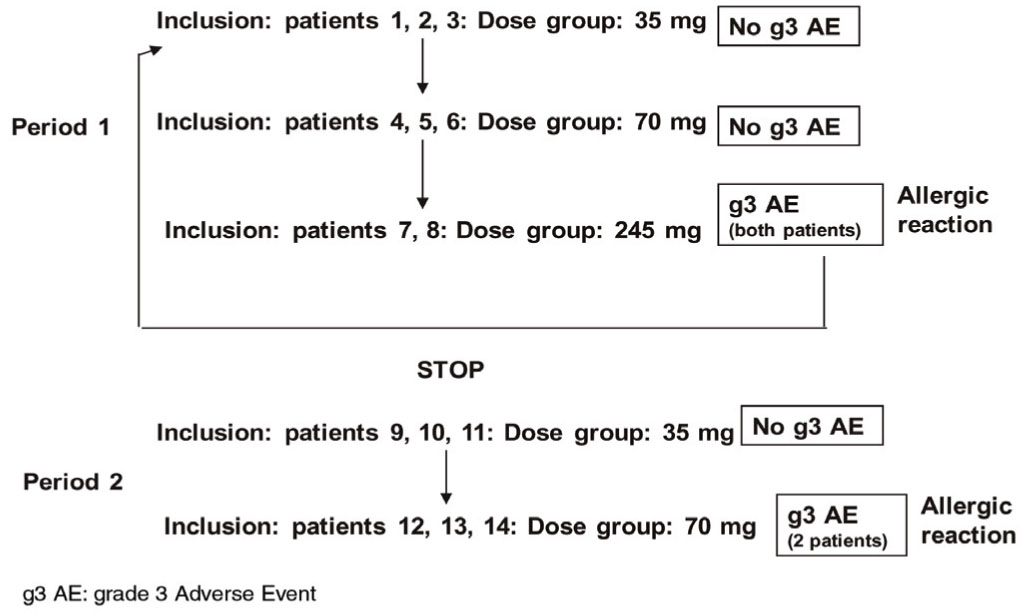
Figure 1 Trial execution.
All the patients completed the CIGB-300 treatment, except those who discontinued treatment after the first dose because of severe adverse events. However regarding patient #12 (Group II), it was impossible to obtain gammagraphic images and to collect serial blood samples because of the severe allergic syndrome. Therefore she was excluded from the biodistribution and pharmacokinetic analyses.
Biodistribution and pharmacokinetic analysis
The comparisons of both biodistribution and pharmacokinetic parameters were performed only between groups of patients who received 35 mg and 70 mg of CIGB-300. Otherwise, data from the two patients who received 245 mg of CIGB-300 were only descriptive.
Gammagraphic images demonstrated tumor uptake of CIGB-300 since 10 min after drug administration in all of the patients. The magnitude of CIGB-300 up taken in tumor and each main target organ is represented in Figure 2. Interestingly, tumor uptake of CIGB-300 was similar for the three dose levels during the period of evaluation (Table 2).
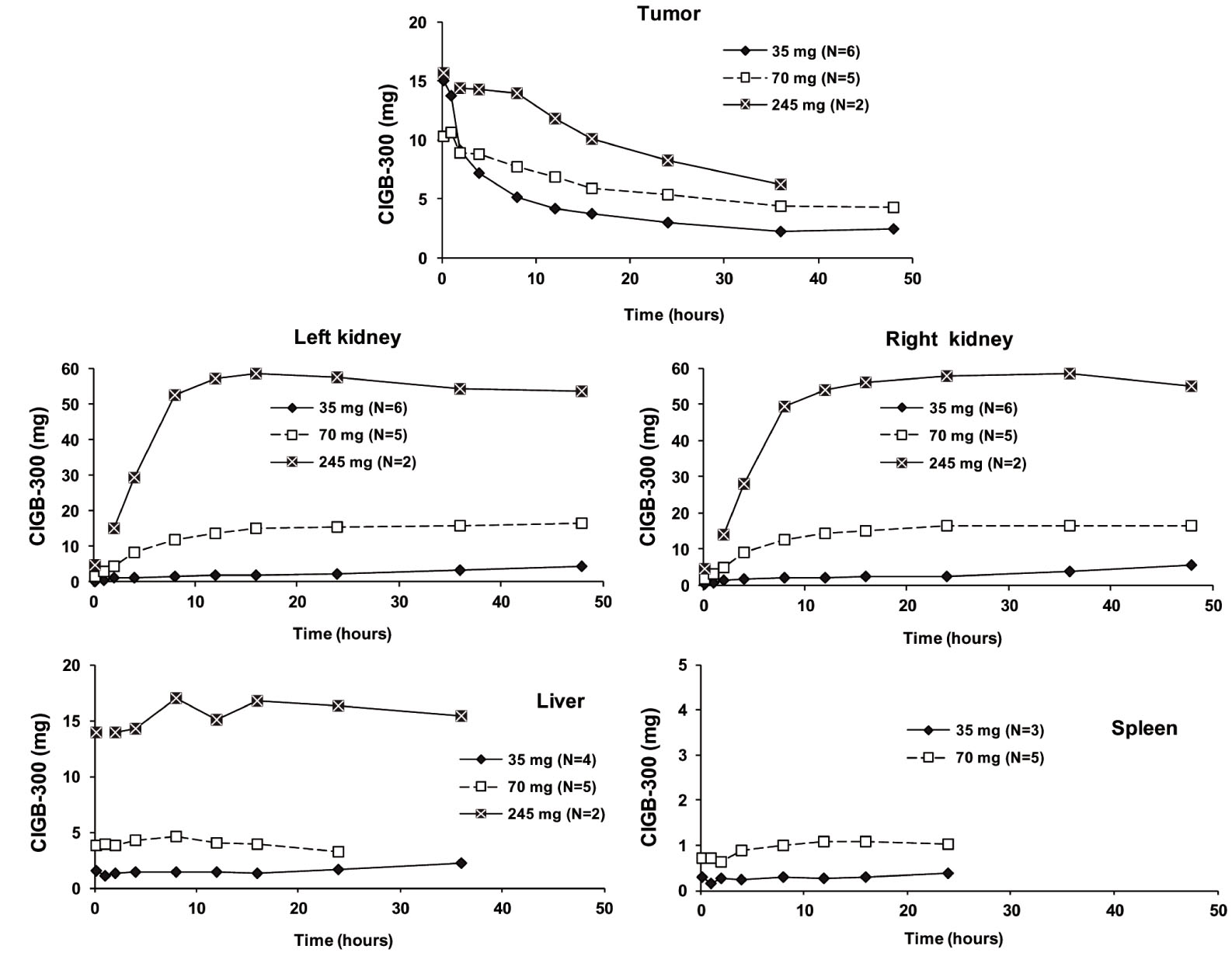
Figure 2 Uptake of CIGB-300 (mg) in the tumor and main target organs determined by gammagraphy: For each dose, average time profiles during the first 48 h after radiolabeled CIGB-300 administration are represented. The different number of patients where specific accumulation occurred at each dose level is shown between parentheses for each organ and tumor. Only one patient demonstrated peptide accumulation in spleen after 245 mg dosage. In one patient (#8) from this group images could not be acquired at 1 h due to technical problems. CIGB-300 was undetectable in tumor and organs of some patients at 36-48 h. For a more easily visualization scales were positioned according to specific organ or tumor uptake. Standard deviations are not shown for the sake of simplicity of the illustration.
| Parameter | Group I (35 mg) N = 6 | Group II (70 mg) N = 5 | p (95% CI) |
| Maximum uptake (%ID) | 43.1 ± 18.4 | 14.8 ± 11.8 | 0.03 |
| (17.2 - 60.8) | (3.2 - 30.0) | (-1.2; 57.8) | |
| Maximum uptake (mg CIGB-300) | 14.9 ± 6.4 | 10.4 ± 8.3 | 0.28 |
| (5.8 - 21.2) | (2.3 - 21.0) | (-10.8; 19.7) | |
| Tumor mass (g)* | 49.1 ± 30.0 | 33.8 ± 21.0 | 0.27 |
| (13.0 - 96.0) | (8.9 - 57.2) | (-34.6; 65.2) | |
| Maximum uptake (mg/g) | 0.38 ± 0.22 | 0.33 ± 0.15 | 0.39 |
| (0.17 - 0.66) | (0.07 - 0.44) | (-0.31; 0.41) | |
| AUC48 (mg*h) | 181 ± 112 | 280 ± 212 | 0.3 |
| (38.6 - 310) | (72.1 - 598) | (-461; 263) | |
| Biological half-life (h) | 4.2 ± 3.2 | 27.4 ± 21.4 | 0.09 |
| (1.6 - 8.0) | (3.0 - 61.9) | (-57.7; 11.3) |
Data are reported as mean ± standard deviation (range);
p: probability that the difference was > 0;
CI: confidence interval for the differences;
*Calculated from MRI initial data
The maximum uptake (10-15 mg) was obtained from 10 min to 1 hr after intratumoral administration, and values gradually decreased until 48 h. Individually, two patients from Group I reached 60% ID (< 20 mg) up taken in the tumor; contrarily, one patient from Group II only reached 3% (2 mg) as observed in Figure 3. Therefore the probability of equality between both groups according to maximum retention expressed as %ID was very low (p = 0.03). These groups were no different neither regarding the maximum CIGB-300 uptake expressed as mg of CIGB-300 nor respect to the uptake corrected by the tumor mass nor AUC48 (mg*h). A trend towards higher values in the Group II was observed for the biological half-life since the probability of equality was lower than 0.1 (Table 2). A significant linear correlation between tumor mass and maximum uptake (mg CIGB-300) was detected (Spearman’s correlation = 0.692, p = 0.009).
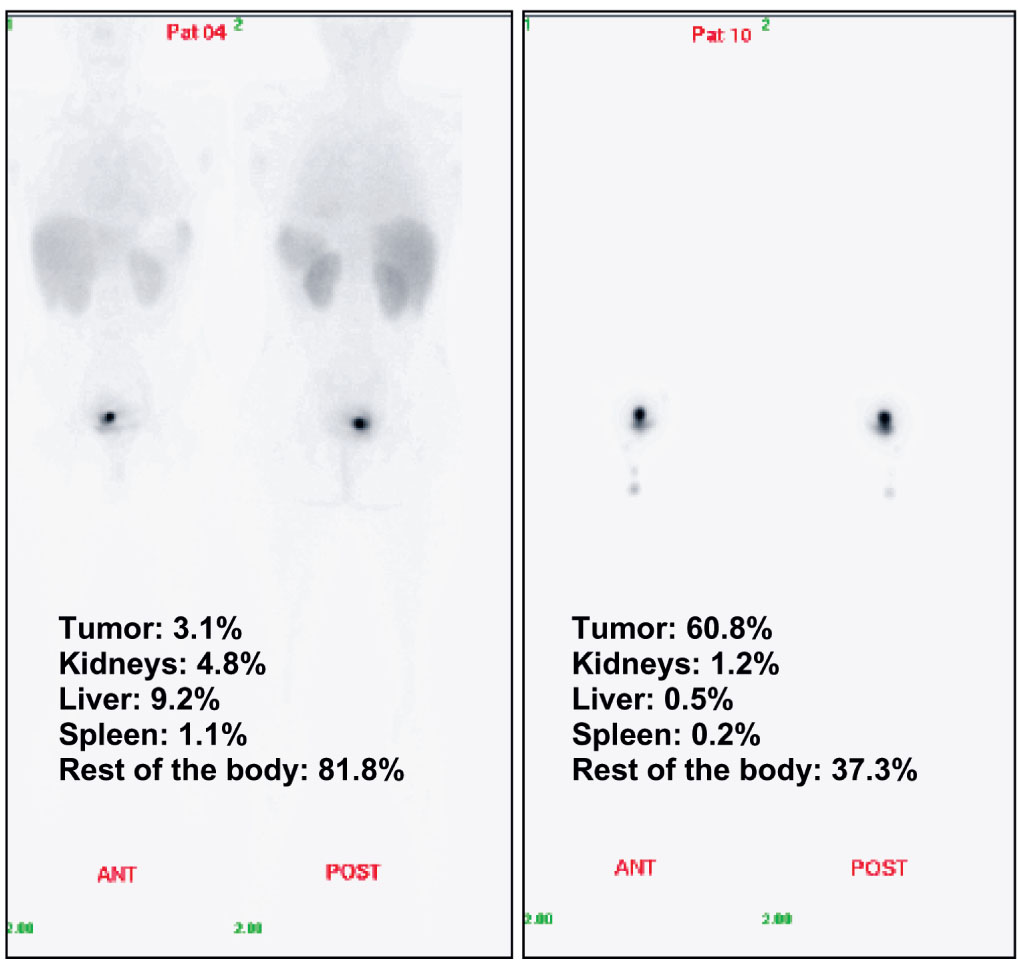
Figure 3 Examples of Whole Body Images at the first time (10 min) of evaluation after intratumoral injection: Individual percentages of the injected dose (%ID) of radioactivity specifically up taken in the tumor and source organs are shown. Patients 10 received 35 mg of 99mTc-labeled CIGB-300 and patient #04 received 70 mg of the same product.
In organs, the highest accumulation occurred in the kidneys with the 245 mg dose as expected, however patients that received 70 mg of CIGB-300 experienced accumulation levels in kidneys several times higher than in those who received 35 mg (Figure 2). No apparent renal dysfunction was observed in any of the patients included in this study. Accumulation of CIGB-300 was also observed at a lesser extent in liver, spleen, heart and lungs. Unspecific accumulation in sensitive organs and other tissues as the brain and bone marrow was not observed.
The pharmacokinetic profiles of CIGB-300 in serum after the first intratumoral injection of 35 or 70 mg are shown in Figure 4. The peptide was rapidly absorbed, reaching its maximum levels in serum at 5-15 min after CIGB-300 administration and gradually decreased until the drug was practically cleared from systemic circulation in 12-24 h. Of note, those patients, who received 70 mg, evidenced higher levels of CIGB-300 during the first hour. Nonetheless, both doses behave similar after 2 h of drug administration. Interestingly, no significant differences on the pharmacokinetic profile were experienced when comparing administration of 35 and 70 mg of CIGB-300 (Table 3). A high inter-individual variability was detected inside each group mainly associated with the reduced number of individuals.
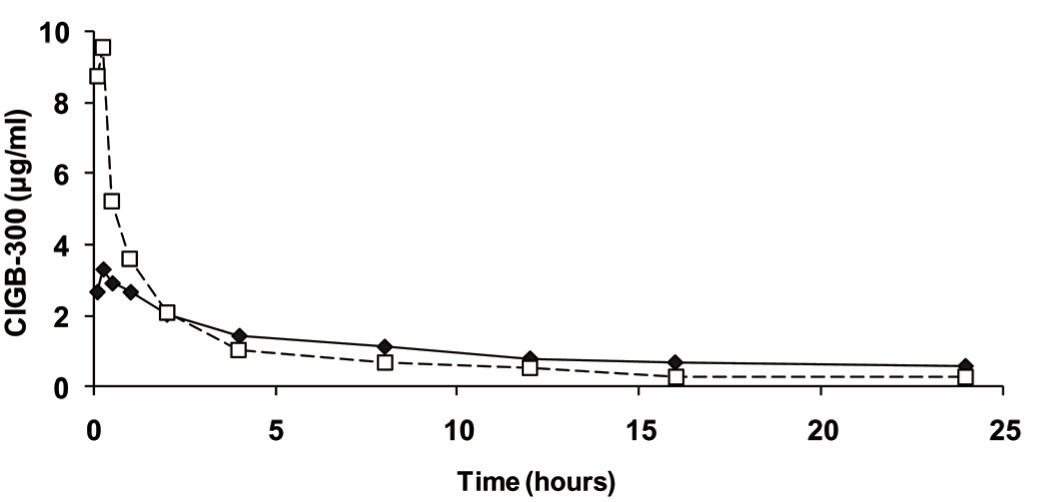
Figure 4 Average CIGB-300 concentration in serum after the first intratumoral administration: Data correspond to cervical cancer patients who received 35 mg (solid line, N = 6) or 70 mg (dashed line, N = 5) of 99mTc-labeled CIGB-300. Concentrations obtained after 245 mg injection were much higher, thus these are not shown. Standard deviations are not shown for the sake of simplicity of the illustration.
| Parameter | Group I (35 mg) N = 6 | Group II (70 mg) N = 5 | p (95% CI) |
| AUC24 (µg*h/mL) | 14.5 ± 49.2 | 19.6 ± 5.2 | 0.56 (-66.9; 56.7) |
| Cmax (µg/mL) | 3.3 ± 6.8 | 7.3 ± 10.8 | 0.66 (-23.0; 15.1) |
| Tmax (h) | 0.75 ± 4.8 | 0.08 ± 0.2 | 0.41 (-5.3; 6.5) |
| l (h-1) | 0.09 ± 0.06 | 0.10 ± 0.05 | 0.57 (-0.12; 0.09) |
| t1/2 (h) | 16.6 ± 16.3 | 9.2 ± 6.6 | 0.26 (-15.4; 30.2) |
| AUCinf (µg*h/mL) | 11.9 ± 91.2 | 20.6 ± 14.0 | 0.56 (-124; 107) |
| Vd/F (L/Kg) | 0.3 ± 2.3 | 0.4 ± 0.7 | 0.53 (-3.2; 3.0) |
| CL/F (L/h*Kg) | 0.04 ± 0.03 | 0.05 ± 0.01 | 0.69 (-0.05; 0.03) |
| MRT24 (h) | 7.0 ± 8.4 | 5.0 ± 3.4 | 0.37 (-9.8; 13.8) |
| MRTinf (h) | 24.2 ± 24.6 | 10.7 ± 7.8 | 0.21 (-19.5; 46.5) |
Data are reported as median ± quartile range, except for λ, t1/2, CL/F and MRTinf expressed as mean ± standard deviation;
p: probability that the difference was > 0;
CI: confidence interval for the differences
The accumulative percentage of CIGB-300 excreted in urine at each time interval for the three tested doses are shown in the Figure 5. During the first 4 h around 25% of the 35 mg dose and 40% of the 245 mg dose were eliminated, but only around 6% of the 70 mg dose of CIGB-300. Since that moment, the elimination rates drastically decreased in the three groups. Therefore, at 24 h, no group reached 50% of total excretion. Median values were 42.4% (14.8 mg) in Group I, 8.7% (6.1 mg) in Group II and 48.3% (118 mg) in Group III. Differences between groups I and II were close to significance (p = 0.052).
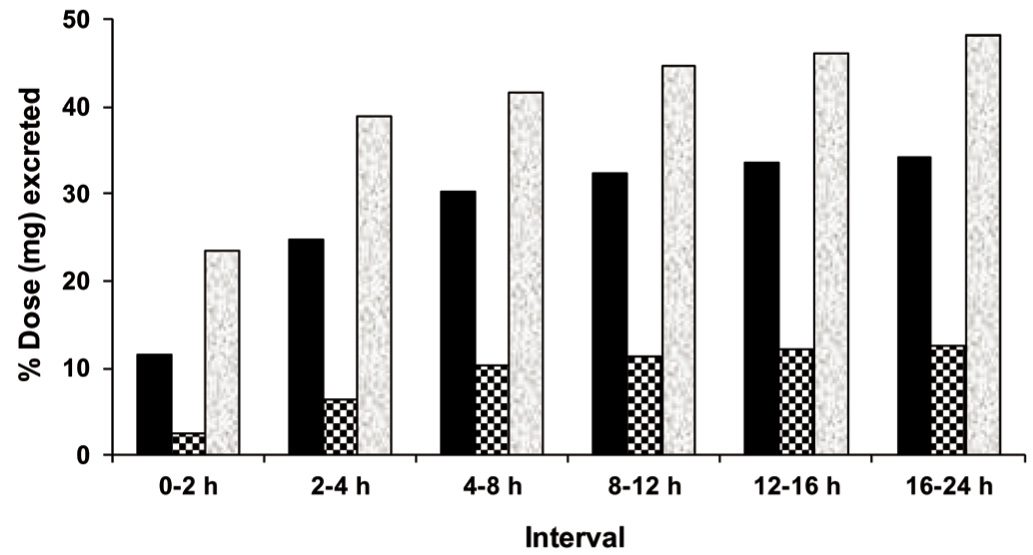
Figure 5 The mean accumulative percentage of the CIGB-300 dose (in milligrams) excreted in urine at each time interval after the first intratumoral administration: Data correspond to cervical cancer patients who received 35 mg (black bars, N = 6), 70 mg (squared bars, N = 5) or 245 mg (shady bars, N = 2) of 99mTc-labeled CIGB-300.
Safety and tolerability
A total of 289 AE were recorded, 247 (85.5%) of them were systemic. More than 70% of the events presented in each group were mild whereas moderate events were presented with the same frequency at the 3 dose levels. The severity of the events had a direct relationship to the administered dose. In the group that received 35 mg of CIGB-300 severe adverse events were not recorded, however in patients treated with 70 mg and 245 mg two grade 3 allergic reactions took place after the first dose in two patients. Therefore, the estimated MTD in this assay was 70 mg, it means the highest dose level at which ≤30% of patients experienced DLT. A higher rate and severity of these events was recorded after the first dose in the first two groups, decreasing with subsequent CIGB-300 administrations (data not shown). Table 4 lists the local and systemic toxicities observed during the study for each dose. Groups I and II were very similar according to type of events. Some of them were absent or had a lower frequency in the Group III as only one dose could be administered. All patients experimented some allergic-like reaction. These reactions included hot flashes (78.6%), flushing (71.4%), and extensive or localized itching, rash or bumps (50% or more) that lasted for a few min to 8 h with a further spontaneous resolution.
| Type of AE | Group I (35 mg) N = 6 | Group II (70 mg) N = 6 | Group III (245 mg) N = 2 | BF (H0Prob) | |
| Local | Pain in lower abdomen | 4 (66.7%) | 5 (83.3%) | 2 (100%) | 0.63 (0.62) |
| Tumor bleeding | 5 (83.3%) | 3 (50.0%) | 0 | 3.87 (0.21) | |
| Vaginal discharge | 2 (33.3%) | 0 | 0 | 0.94 (0.52) | |
| Systemic | Allergic reaction | 6 (100%) | 6 (100%) g3: 2 (33.3%) |
g3: 2 (100%) | 0.16 (0.87) |
| Hot flashes | 6 (100%) | 5 (83.3%) | 0 | 9.39 (0.10) | |
| Headache | 5 (83.3%) | 4 (66.7%) | 1 (50.0%) | 0.86 (0.54) | |
| Flushing | 4 (66.7%) | 5 (83.3%) | 1 (50.0%) | 0.86 (0.54) | |
| Tachycardia | 4 (66.7%) | 4 (66.7%) | 2 (100%) | 0.69 (0.59) | |
| Extensive itching | 3 (50.0%) | 3 (50.0%) | 2 (100%) | 1.16 (0.46) | |
| Extensive rash | 3 (50.0%) | 3 (50.0%) | 1 (50.0%) | 0.66 (0.60) | |
| Localized rash | 3 (50.0%) | 3 (50.0%) | 0 | 1.16 (0.46) | |
| Conjunctival injection | 2 (33.3%) | 2 (33.3%) | 2 (100%) | 2.07 (0.33) | |
| Hypotension | 1 (16.7%) | 4 (66.7%) | 1 (50.0%) | 2.58 (0.28) | |
| Extensive cramps | 2 (33.3%) | 2 (33.3%) | 1 (50.0%) | 0.69 (0.59) | |
| Localized itching | 4 (66.7%) | 1 (16.7%) | 0 | 3.44 (0.23) | |
| Localized facial edema | 2 (33.3%) | 2 (33.3%) | 0 | 0.69 (0.59) | |
| Extensive bumps | 2 (33.3%) | 1 (16.7%) | 0 | 0.63 (0.62) | |
| Vomiting | 2 (33.3%) | 1 (16.7%) | 0 | 0.63 (0.62) | |
| Nausea | 1 (16.7%) | 0 | 1 (50.0%) | 1.17 (0.46) | |
| Retrosternal pain | 1 (16.7%) | 1 (16.7%) | 0 | 0.08 (0.93) | |
| Vasovagal syndrome | 0 | 0 | 1 (50.0%) | 1.08 (0.48) |
Data are presented as number of patients with each adverse reaction (%); BF: Bayes’ factor toward the hypothesis of dependency; (H0 Prob): Probability of independency; g3: grade 3 adverse event; Other local (edema, ardor) and systemic (localized cramps and bumps, hypertension, dizziness, bradycardia, tremors, dyspnea, asthenia, distal cyanosis, palpebral edema, sleepiness, ringing in the ears, palpitations, extrasystole, hoarseness, adiamic, bronchospasms, dry mucosa) AE were only observed in one patient each one
Interestingly, the adverse events classified as “allergic reaction" [17] had a significant correlation with the histamine levels in plasma and these in turn were related to the CIGB-300 concentration (Table 5). Thus, the allergic reactions grade 3 was identified as the DLT in this study.
| Variable | Spearman R value | p |
| CIGB-300* | 0.721 | 0.000001 |
| Grade AR** | 0.621 | 0.041 |
AR: Allergic reaction;
*Correlation was significant at the 0.01 level (2-tailed);
**Correlation was significant at the 0.05 level (2-tailed) 15 min after injection
The cardiovascular events such as tachycardia, hypotension, hypertension and palpitations were mild and had a rapid and spontaneous resolution. The most common local events in the genital apparatus were pain in lower abdomen (78.6%) and tumor bleeding (57.1%) without necessity of further treatment.
Vital signs remained within normal ranges except for the reduction in heart beats and blood pressure during the first hour after the first application in the group that received the maximum dose. There were no differences between baseline and post-treatment blood counts and serum chemistry results in each group (data not shown).
Biological effect of CIGB-300
The immunohistochemical analysis of B23/NPM was performed only in those patients who fulfilled the drug regimen (10 patients). Interestingly, CIGB-300 treatment reduced B23/NPM levels in the nucleolus of cervical tumor cells in 7/10 evaluated patients (p = 0.034). A representative picture of the reduction of the B23/NPM nucleolar expression after CIGB-300 treatment is provided in Figure 6. Otherwise, CK2β levels were not modulated by the CIGB-300 treatment as demonstrated by immunohistochemistry (data not shown).
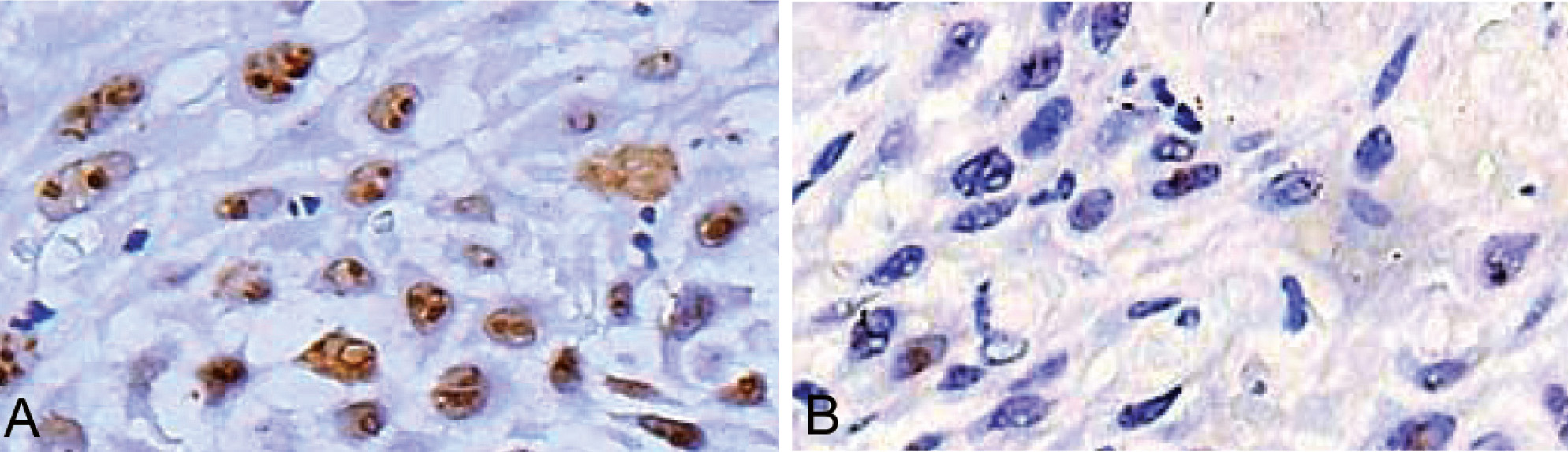
Figure 6 Representative pictures of the B23/NPM expression in cervical tumors: Paraffin-embedded tumor biopsies were deparaffined and further processed for immunohistochemistry as described in material and methods. Finally, biopsies were stained with peroxidase stain and further contrasted with hematoxylin. A and B represent the typical baseline and post-CIGB-300 treatment levels of B23/NPM, respectively.
DiscussionTop
The design of this study was conceived to explore the tumor uptake, MTD, DLT, pharmacokinetics and signs of biological effect of CIGB-300 neoadjuvant to chemoradiotherapy in women with cervical cancer stage IB2/II.
Data obtained from the biodistribution analysis demonstrated that intratumor injection of CIGB-300 leads to a high tumor uptake which is markedly superior to those achieved for other cancer targeted therapies systemically administered. That is the case of monoclonal antibodies where <1% of the antibody reach the tumor [20]. Interestingly, the percent of injected drug in this study was lower as the CIGB-300 increased hence leading to a high accumulation in source organs (e.g. kidneys). It means those patients who received 35 mg of the drug showed higher tumor uptake than those ones received 70 mg (43% vs.15%). However, no major differences were observed in the total amount of CIGB-300 uptake in the tumor per se when comparing both doses. Such a finding leads to the speculation that a putative “saturation” of the tumor uptake of CIGB-300 in that dose range could take place. Such hypothesis needs to be clarified in further clinical and non-clinical studies.
Nonetheless, other factors related to the way of delivery could strongly influence on the tumor uptake. These factors are the volume of administration, in this case 2 ml, the injection at a single site and the time of delivery (3-5 min). The actual impact of such a factors in the tumor uptake of CIGB-300 merits further confirmation.
As expected for cationic peptides and verified in previous unpublished non-clinical studies with CIGB-300, it seems to be excreted by the kidney and liver. Importantly, despite CIGB-300 trended to accumulate in kidneys as determined at 24 h after the first administration, renal toxicity was no detected at short or middle term in any patient.
The low levels of CIGB-300 detected in urine at 24 h after the first administration were coincident with the renal accumulation observed in that experimental point. Therefore, these findings suggest that the peptide was not completely excreted from the body in 24 h after a single injection of 35 and 70 mg of CIGB-300.
The pharmacokinetic profile of CIGB-300 showed a close relation to the whole body distribution, mainly in the tumor. The higher Cmax obtained in blood with 70 mg compared to 35 mg indicates a more profound and rapid escape from the site of administration toward blood stream.
Data collected in this study permit to assume that 35 and 70 mg of CIGB 300 are safe when injected intratumorally in patients with cervical cancer stage IB2/II, and that 70 mg can be considered as the MTD in this clinical setting. The appearance of allergic reactions after administration of anticancer drugs, including kinase inhibitors, has been previously reported [21, 22]. However, the systemic "allergic-like" events observed after CIGB-300 treatment were transient and well-controlled. Such events were caused by the histamine release in the blood stream and its severity did correlate with the histamine levels. This syndrome could be also related to CIGB-300's molecular structure, composed by several predominantly positively charged amino acids that make it a very basic peptide and, therefore, behave as a potential histamine releaser [23]. The prophylactic use of antihistamine medication in cancer patients under treatment with these or higher CIGB-300 doses should be studied in future clinical trials. On the other hand, the single vasovagal event reported does not seem to be drug-related but rather associated with the mode of application. This event previously presented during administration of this product [14] has been also reported during uterine cervix manipulation with different techniques [24].
Despite this study was not designed to evaluate the therapeutic efficacy as the primary objective, we aimed to look for a putative response biomarker linked to the mechanism of action of CIGB-300 as suggestive of clinical activity. As a molecular targeted agent, CIGB-300 inhibits the CK2-mediated phosphorylation of the B23/NPM nucleolar protein and this event lead to apoptosis in tumor cells. Furthermore, degradation of B23/NPM on CIGB-300-treated cells has been also documented in non-small cell lung cancer cells with great sensitivity toward CIGB-300 [10, 11]. Therefore, the decrease on the B23/NPM might be indicative of some clinical activity at the molecular level. Interestingly, our data suggest an overall non-dose related reduction in B23/NPM levels in tumors. These findings are in line with those from biodistribution in which a plateau of the tumor uptake was observed for 35 and 70 mg of CIGB-300. The evaluation of B23/NPM in future Phase 2 clinical trials with CIGB-300 could help to monitor molecular changes that could be important in controlling tumor growth.
ConclusionTop
Altogether, here we have demonstrated that CIGB-300 can achieve high levels of tumor uptake when locally delivered by intratumoral injections. This route of administration is safe with an MTD of about 70 mg and the histaminergic reactions as the DLT. The doses of 35 and 70 mg of CIGB-300 reach a plateau in terms of tumor uptake and molecular events induced by this peptide in tumor biopsies. Although not designed for efficacy, there were signals of activity associated with CIGB-300 administration in this study. Therefore, CIGB-300 represents a novel peptide-based drug that merits to be tested in future Phase 2 clinical trials in patients with cervical cancer stage IB/II before going to chemoradiotherapy.
Acknowledgements
The authors wish to thank the doctors Sergio Martínez, Jorge Rodriguez and Luis Castillo and the nurses Rosalina Falcón, and Tec. Kalep Ventura, Lic. Alina Pérez, for their participation in the clinical work. They also thank Ángela Tuero, Yunia Delgado, Cimara Bermúdez, Grettel Melo, Ketty Cruz, María A Delgado, Silvia Barcelona, Marisol Cruz, for their assistance and Matilde López, Osvaldo Acosta, Hilda Garay for their contribution in the drug characterization and formulation. The authors received CIGB-300 peptide free from Heber Biotec, Havana, Cuba.
Funding
This work was supported by HeberBiotec S.A, Havana (products, insurance, reagents), by Biorec, and by the Ministry of Public Health of Cuba (hospital facilities and general medical care of the patients).
Conflict of interest
The authors wish to express that they have no conflict of interest.
ReferencesTop
[1] Castellsagué X (2008) Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 110: S4-S7. Article Pubmed
[2] Grigsby PW (2001) Cervical cancer: combined modality therapy. Cancer J 7: S47-S50. Pubmed
[3] Tang J, Tang Y, Yang J, Huang S (2012) Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol Oncol 125: 297-302. Article Pubmed
[4] Huang EY, Wang CJ, Hsu HC, Hao Lin, Chen HC, et al. (2004) Dosimetric factors predicting severe radiation-induced bowel complications in patients with cervical cancer: combined effect of external parametrial dose and cumulative rectal dose. Gynecol Oncol 95: 101-108. Article Pubmed
[5] Symonds RP, Collingwood M, Kirwan J, Humber CE, Tierney JF, et al. (2004) Concomitant hydroxyurea plus radiotherapy versus radiotherapy for carcinoma of the uterine cervix: a systematic review. Cancer Treat Rev 30: 405-414. Article Pubmed
[6] Baselga J (2006) Targeting Tyrosine Kinases in Cancer: The Second Wave. Science 312: 1175-1178. Article Pubmed
[7] Sebolt-Leopold JS, English JM (2006) Mechanisms of drug inhibition of signaling molecules. Nature 441: 457-462. Article
[8] Arkenau HT, Plummer R, Molife LR, Olmos D, Yap TA, et al. (2012) A phase I dose escalation study of AT9283, a small molecule inhibitor of aurora kinases, in patients with advanced solid malignancies. Ann Oncol 23: 1307-1313. Article Pubmed
[9] Perea SE, Reyes O, Puchades Y, Mendoza O, Vispo NS, et al. (2004) Antitumor effect of a novel proapoptotic peptide that impairs the phosphorylation by the protein kinase 2 (casein kinase 2). Cancer Research 64: 7127-7129. Article Pubmed
[10] Perea SE, Reyes O, Baladron I, Perera Y, Farina H, et al. (2008) CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Mol Cell Biochem 316: 163-167. Article Pubmed
[11] Perera Y, Farina HG, Gil J, Rodriguez A, Benavent F, et al. (2009) Anticancer peptide CIGB-300 binds to nucleophosmin/B23, impairs its CK2-mediated phosphorylation, and leads to apoptosis through its nucleolar disassembly activity. Mol Cancer Ther 8: 1189-1196. Article Pubmed
[12] Perea SE, Baladron I, Garcia Y, Perera Y, Lopez A, et al. (2011) CIGB-300, a synthetic peptide-based drug that targets the CK2 phosphoaceptor domain. Translational and clinical research. Mol Cell Biochem 356: 45-50. Article Pubmed
[13] Perera Y, Farina HG, Hernández I, Mendoza O, Serrano JM, et al. (2008) Systemic administration of a peptide that impairs the Protein Kinase (CK2) phosphorylation reduces solid tumor growth in mice. Int J Cancer 122: 57-62. Article Pubmed
[14] Solares AM, Santana A, Baladrón I, Valenzuela C, González CA, et al. (2009) Safety and preliminary efficacy data of a novel Casein Kinase 2 (CK2) peptide inhibitor administered intralesionally at four dose levels in patients with cervical malignancies. BMC Cancer 9: 146. Article Pubmed
[15] Benedet JL, Hacker NF, Ngan HY (2003) FIGO Committee on Gynecologic Oncology, IGCS Guidelines Committee. Staging classification and clinical practice guidelines of gynecologic cancer, 2nd edn. pp. 36–40, FIGO Website: http://www.figo.org/publications. Book
[16] Carter RE, Woolson RF (2004) Statistical design considerations for pilot studies transitioning therapies from the bench to the bedside. J Transl Med 2: 37-39. Article Pubmed
[17] Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (2003) Version 3.0, DCTD, NCI, NIH, DHHS. March 31, ctep Website: http://ctep.cancer.gov/forms/CTCAEv3.pdf. Article
[18] Naranjo CA, Shear NH, Busto U (1998) Adverse drug reactions. In: Kalant H, Roschlau WHE, editors. Principles of medical pharmacology, 6th edn. New York: Oxford University Press pp. 791–800.
[19] Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, et al. (1999) MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med 40: 37S-61S. Article Pubmed
[20] Jain M, Venkatraman G, Batra SK (2007) Optimization of radioimmunotherapy of solid tumors: biological impediments and their modulation. Clin Cancer Res 13: 1374-1382. Article Pubmed
[21] Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, et al. (2007) Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 13: 3913-3921. Article Pubmed
[22] Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, et al. (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357: 2040-2048. Article Pubmed
[23] Watt AP (2001) Mast cells and peptide induced histamine release. Inflammopharmacology 9: 421-434. Article
[24] Droegemuller W, Herbst AL, Michell DR (1987) Comprehensive Gynecology. St Louis, MO: CV Mosby Co pp. 48–51.
Appendix
The other members of the CERVIFARM-300 Group are: Robin García-Diéguez, (“Hermanos Ameijeiras” Hospital), Zaida Barbón-Gassó, Dagnelia Castillo-Jones (“Ramón González Coro” Hospital), Alba Vaillant-Baralt (Central Clinic “Cira García”), Alejandro Linchenat-Lambert (National Institute of Oncology, Havana), Julio Mora-Valle, Kenia Aguilar-Fabré (Gyneco-obstetric Teaching Hospital “Eusebio Hernández”, Havana), María de los Á Castañeda-Capote, Martha López (Pediatric Hospital Ángel A Aballí), Regla Fang-Mederos, Doris González-Díaz (“Gustavo Aldereguía” Hospital, Cienfuegos), Águeda Santana-Martínez (Gyneco-obstetric Teaching Hospital 10 de Octubre, Havana), Sonia Fanjul (Gyneco-obstetric Teaching Hospital “Mariana Grajales”, Santa Clara), Víctor Salgueiro-Medina (“Abel Santamaría” Hospital, Pinar del Río), Leonardo González-Obregón, Isabel Álvarez-Sánchez (“Carlos Manuel de Céspedes” Hospital, Bayamo), Leovaldo Álvarez-Falcón, Julio Baldomero-Hernández (Clinical Investigation Department, CIGB, Havana).


