Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 2, Issue 2, February 2014, Pages 36–39
Original researchOpen Access
Usefulness of the Glasgow prognostic score for predicting survival in patients with resected non-small cell lung cancer
- 1 Department of Surgery II, Faculty of Medicine, University of Miyazaki, Kihara 5200, Kiyotake, Miyazaki, 889-1692, Japan
*Corresponding author:Masaki Tomita, MD, Department of Surgery II, Faculty of Medicine, University of Miyazaki, Kihara 5200, Kiyotake, Miyazaki, 889-1692, Japan. Tel: +81 985852291; Fax: +81 985855563; Email: mtomita@med.miyazaki-u.ac.jp
Received 31 October 2013 Revised 5 January 2014 Accepted 14 January 2014 Published 23 January 2014
DOI: http://dx.doi.org/10.14312/2052-4994.2014-5
Copyright: ©2014 Tomita M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Background: Few studies have investigated whether the Glasgow prognostic score (GPS), an inflammation based prognostic score, is useful for postoperative prognosis of non-small cell lung cancer (NSCLC). Materials and methods: Two hundred and eighty nine consecutive patients of resected NSCLC with a follow-up period more than 5 years were enrolled. GPS was calculated on the basis of admission data as follows: patients with elevated C-reactive protein level (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) were assigned to GPS 2. Patients with one or no abnormal value were assigned to GPS 1 or GPS 0. Results: Study patients were allocated as follows: 244 (84.44 %) to GPS 0, 28 (9.69 %) to GPS 1, and 16 (5.54 %) to GPS 2. The prognosis of the patients with GPS 2 was significantly poorer. Multivariate logistic analysis identified age, gender, pT status, pN status, serum CEA level and GPS were found to be independent prognostic variables. Conclusions: Preoperative GPS, especially GPS 2, may be useful for postoperative prognosis of patients with NSCLC.
Keywords: Glasgow prognostic score; albumin; CRP; non-small cell lung cancer; prognosis
IntroductionTop
It is increasingly recognized that it is not only the intrinsic properties of tumor cells that determine tumor spread but also the host inflammatory response [1, 2]. The presence of a preoperative systemic inflammatory response in the patient has predicted poor survival after resection in a number of cancers [1-2]. The host systemic inflammatory response can be assessed by examining the changes in the circulating concentrations of acute-phase reactants, such as elevated concentrations of C-reactive protein (CRP) and low concentrations of albumin [3]. It is of interest that either individually or combined, these factors have been shown to be stage- and performance status-independent prognostic factors in patients with a variety of inoperable cancers [3-8].
Forrest and coworkers in Glasgow reported an inflammation based prognostic score reflecting the presence of a systemic inflammatory response, as evidenced by the combination of an elevated C-reactive protein (CRP) level and hypoalbuminemia [3]. This systemic inflammation based prognostic score was subsequently termed the Glasgow prognostic score (GPS).
There is increasing evidence that GPS is an independent prognostic factor in patients with a variety of cancer [4-6]. For non-small cell lung cancer (NSCLC), some studies reported the prognostic significance of GPS in patients with advanced disease [3, 7, 8].
Since there have been no data concerning the usefulness of the GPS as a predictor of prognosis in patients who underwent radical resection of NSCLC, the present study was conducted to investigate whether GPS could predict postoperative survival in patients with NSCLC.
Patients and methodsTop
Patients
Two hundred eighty nine NSCLC patients who underwent surgery from 2003 to 2007 in our hospital and for whom preoperative laboratory data for CRP and albumin were available were enrolled into the present retrospective study. Patients who did not receive complete resection and who had a follow-up period of less than 5 years were excluded.
Laboratory tests
The clinical investigation section of our hospital measured preoperative serum CRP and albumin level as part of routine preoperative examination. For evaluation of the GPS, the results of blood were used. GPS was constructed as previously described [3-5]. Patients with both an elevated CRP (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) were allocated a score of 2 (GPS 2). Accordingly, patients with neither of these abnormalities were allocated a score of 0 (GPS 0). Remaining patients in whom only one of the biochemical parameter was abnormal were assigned a score of 1 (GPS 1). Pathological (p) tumor-node-metastasis (TNM) staging was recorded in all patients based on the 7th edition of the American Joint Committee on Cancer (AJCC)/ International Union Against Cancer (UICC) classification. The baseline characteristics are summarized in Table 1. Follow-up information, including cause of death, was ascertained through a review of clinic notes and direct or family contact.
| GPS 0 (%) | GPS 1 (%) | GPS 2 (%) | p Value | ||
| Age | <65 | 87 (90.63) | 5 (5.21) | 4 (4.17) | 0.128 |
| ≥65 | 157 (81.77) | 23 (11.98) | 12 (6.25) | ||
| Gender | Male | 148 (83.15) | 19 (10.67) | 11 (6.18) | 0.638 |
| Female | 96 (87.27) | 9 (8.18) | 5 (4.55) | ||
| Histology | Adenocarcinoma | 183 (84.72) | 21 (9.72) | 12 (5.56) | 1 |
| Others | 61 (84.72) | 7 (9.72) | 4 (5.56) | ||
| pStage | I | 180 (86.54) | 19 (9.13) | 9 (4.33) | 0.274 |
| IIIII | 64 (80.00) | 9 (11.25) | 7 (8.75) | ||
| pT status | pT1 | 150 (85.23) | 16 (9.09) | 10 (5.68) | 0.899 |
| pT2-4 | 94 (83.93) | 12 (10.71) | 6 (5.36) | ||
| pN status | pN0 | 192 (86.88) | 20 (9.05) | 9 (4.07) | 0.094 |
| pN1-2 | 52 (77.61) | 8 (11.94) | 7 (10.45) | ||
| CEA | Normal | 161 (84.29) | 21 (10.99) | 9 (4.71) | 0.431 |
| High | 83 (85.57) | 7 (7.22) | 7 (7.22) | ||
| Adjuvant | Performed | 16 (69.57) | 3 (13.04) | 4 (17.39) | 0.095 |
| Not performed | 228 (86.04) | 25 (9.43) | 12 (4.53) | ||
Abbrevations: GPS: Glasgow Prognostic Score; CEA: Carcinoembryionic antigen.
Statistical analysis
The chi-square test with Yates’ correction was used to correlate clinicopathological parameters with the GPS. The survival curves of the patients were plotted by using the Kaplan–Meier method and analyzed using the log-rank test. Cox regression hazard model was used for univariate and multivariate analyses to assess the prognostic value of GPS. Statistical calculations were conducted with JMP (SAS Institute Inc. Cary, NC, USA) and values of p less than 0.05 were accepted as being significant.
ResultsTop
Relationship between GPS and clinicopathologic factors
Study patients were allocated as follows: 244 (84.44 %) to GPS 0, 28 (9.69 %) to GPS 1, and 16 (5.54 %) to GPS 2. Relationships between GPS and clinicopathologic features are shown in Table 1. Age, gender, histology, pStage, pT status, pN status and serum carcinoembryonic antigen (CEA) level showed no significant relationship with GPS classification. In the present series, 23 patients were received the adjuvant therapies, and there was also no relationship between GPS and adjuvant therapies (performed vs. not performed, Table 1).
GPS and patients’ survival
The 5-year survival rates of patients with GPS 0, GPS 1 and GPS 2 were 73.8%, 67.9%, and 31.6%, respectively (Figure 1). The 5-year survival of patients with GPS 2 was significantly poor (p<0.0001). However we failed to find the difference in patients’ survival between GPS 0 and GPS 1 (p=0.556).
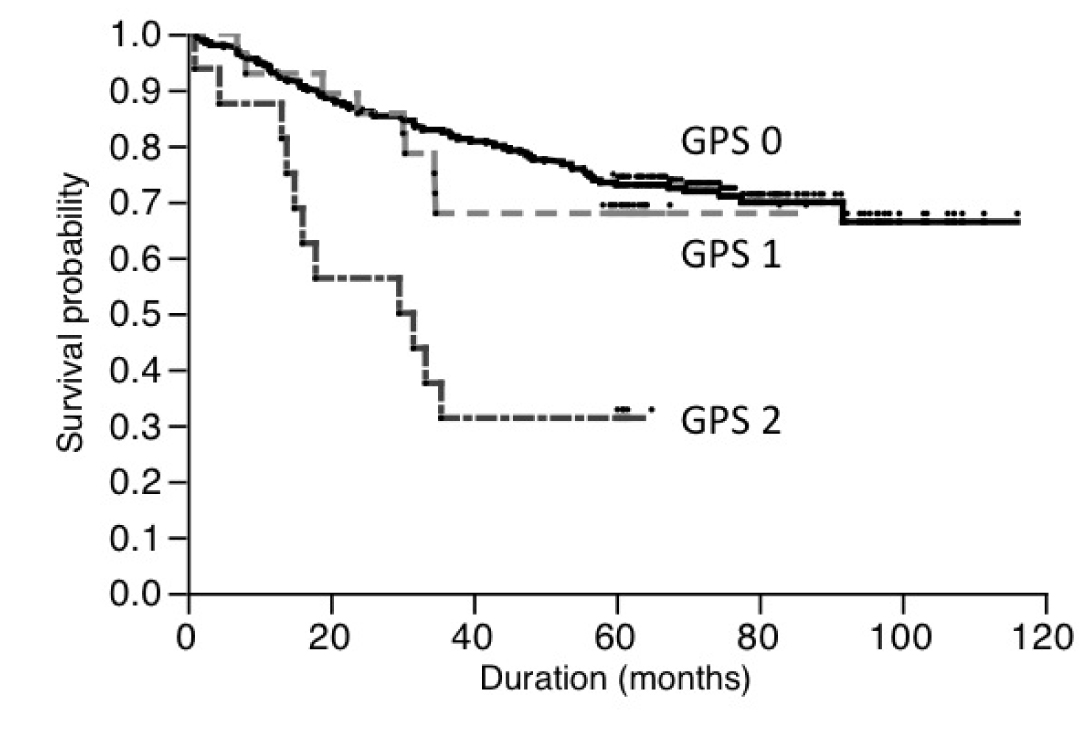
Figure 1 Survival of patients based on Glasgow prognostic score.
Univariate Cox proportional hazard regression analysis revealed that age (<65 vs. ≥65), the gender (male vs. female), the histological subtype (adenocarcinoma vs. others), pT status (pT1 vs. pT2-3), pN status (pN0 vs. pN1-2), serum CEA level (normal vs. high) and GPS (GPS 0-1 vs. GPS 2) were related to patients’ prognoses (Table 2). Since the number of patients with GPS 2 was small, we also examined using as follows: GPS 0 vs. GPS 1-2. GPS was also found to be a significant prognostic factor when we compared between patients with GPS 0 and GPS 1-2 (Table 2). Multivariate analyses using the same factors as those in Table 2 revealed that age (<65 vs. ≥65), the gender (female vs. male), pT status (pT1 vs. pT2-3), pN status (pN0 vs. pN1-2), serum CEA level (normal vs. high) and GPS (GPS 0-1 vs. GPS 2) were the independent predictors of survival (Table 3A). As shown in Table 3B, GPS was also an independent prognostic factor when compared between patients with GPS 0 and GPS 1-2.
| Favorable | Unfavorable | Risk ratio | 95% CI | p Value | |
| Age | <65 | ≥65 | 0.440311 | 0.255-0.721 | 0.0008 |
| Gender | Female | Male | 0.3829776 | 0.227-0.617 | <0.0001 |
| Histology | Adenocarcinoma | Others | 0.6255504 | 0.406-0.986 | 0.0434 |
| pT status | pT1 | pT2-3 | 0.2827077 | 0.182-0.431 | <0.0001 |
| pN status | pN0 | pN1-2 | 0.3090869 | 0.204-0.472 | <0.0001 |
| serum CEA | Normal | High | 0.3772665 | 0.249-0.570 | <0.0001 |
| GPS | GPS 0-1 | GPS 2 | 0.2586999 | 0.143-0.517 | 0.0004 |
| GPS | GPS 0 | GPS 1-2 | 0.5064898 | 0.313-0.857 | 0.0124 |
Abbreviations: CI: Confidence interval; CEA: carcinoembryonic antigen; GPS: Glasgow prognostic score.
| Favorable | Unfavorable | Risk ratio | 95% CI | p Value | |
| Age | <65 | ≥65 | 0.5484868 | 0.314-0.912 | 0.0196 |
| Gender | Female | Male | 0.5350206 | 0.312-0.879 | 0.0128 |
| Histology | Adenocarcinoma | Others | 0.8156658 | 0.519-1.309 | 0.3912 |
| pT status | pT1 | pT2-3 | 0.3905487 | 0.245-0.614 | <0.0001 |
| pN status | pN0 | pN1-2 | 0.4739423 | 0.304-0.744 | 0.0013 |
| serum CEA | Normal | High | 0.578387 | 0.375-0.891 | 0.0131 |
| GPS | GPS 0-1 | GPS 2 | 0.2395021 | 0.130-0.485 | 0.0003 |
| Favorable | Unfavorable | Risk ratio | 95% CI | p Value | |
| Age | <65 | ≥65 | 0.5648492 | 0.322-0.942 | 0.0281 |
| Gender | Female | Male | 0.5220322 | 0.304-0.858 | 0.0095 |
| Histology | Adenocarcinoma | Others | 0.8482973 | 0.538-1.367 | 0.4913 |
| pT status | pT1 | pT2-3 | 0.4247833 | 0.267-0.667 | 0.0002 |
| pN status | pN0 | pN1-2 | 0.4783194 | 0.305-0.756 | 0.0017 |
| serum CEA | Normal | High | 0.5357183 | 0.344-0.833 | 0.0056 |
| GPS | GPS 0 | GPS 1-2 | 0.5377099 | 0.327-0.921 | 0.0249 |
Abbreviations: CI: Confidence interval; CEA: carcinoembryonic antigen; GPS: Glasgow prognostic score.
DiscussionTop
In the present study, we investigated the impact of a systemic inflammatory response, reflected in GPS, on the prediction of outcomes after curative resection of NSCLC. Since Forrest and coworkers [3] first published a scoring system based on the combination of CRP and albumin in patients with inoperable NSCLC, there is now increasing evidence for a role of the systemic inflammatory response in predicting survival in various cancers, independent of tumor stage [4-6]. Although some previous works examined the patients with resectable cancer [5, 6], to our knowledge, none of these studied resectable NSCLC.
Our results showed the prognostic significance of GPS in resectable NSCLC and the results of our study are consistent with these previous works [3-8].
GPS is thought to reflect systemic inflammatory response in the tumor microenvironment. The cause-effect relationship between a systemic inflammatory response and cancer survival is questionable. However, it may be that the presence of a systemic inflammatory response and the associated nutritional decline influences tolerance [9]. The mechanism of up- regulation of CRP is controlled by cytokines, such as interleukin-6 (IL-6), IL-8 and tumor necrosis factor-alpha, and high levels of CRP might reflect an increased level of IL-6 in patients with cancer s[10].
Hypoalbuminemia has been also known to be a consequent presentation caused by systemic inflammation [4] and previous reports have shown that the development of hypoalbuminemia is likely to be a secondary event following serum elevation of CRP [11]. Moreover, some previous works demonstrated that most tumor-bearing patients with hypoalbuminemia already had serum elevations of CRP [11]. Accordingly, hypoalbuminemia and high level of CRP may be regarded as a paraneoplastic phenomenon.
The number of patients with GPS 1 and 2 in the present study were very small when compared previous works [3-8]. Many patients enrolled in previous works had advanced disease, possibly causing malnutrition, whereas we examined the patients with resectable disease. Therefore, the reason for the difference in the frequency of GPS 1 and 2 might be due to differences in stage of patients’ population. In other words, the present study focused on resectable NSCLC patients, whereas most previous works included many advanced stage patients. Furthermore we failed to find the difference in survival between patients with GPS 0 and 1, and one of most possible reason might be also due to the small number of patients with GPS 1. Nozoe et al. [12] proposed other cut-off values for albumin and CRP, which are used to determine abnormality in their laboratory system, for gastric cancer. Therefore there is a possibility that another more useful cut-off value for resectable NSCLC might exist. Further studies in this area are warranted.
Currently, blood test for serum albumin and CRP level are inexpensive and routine determinations. Thus GPS should be included in routine preoperative clinical assessment and treatment planning. The elevated preoperative GPS seems to be an independent predictor of poor outcome for resectable NSCLC patients and, therefore, may be considered better stratification factor in future clinical trials.
ConclusionTop
Preoperative GPS could be used to identify a subgroup of patients with NSCLC who are eligible for radical resection but show poor prognosis.
Conflict of interest
All the authors declare that they have no conflict of interest.
ReferencesTop
[1] Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545. Article Pubmed
[2] Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867. Article Pubmed
[3] Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ (2003) Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 89:1028-1030. Article Pubmed
[4] McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, et al. (2001) Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer 41:64–69. Article Pubmed
[5] McMillan DC, Canna K, McArdle CS (2003) Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg 90:215–219. Article Pubmed
[6] Roxburgh CS, McMillan DC (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6:149-163. Article Pubmed
[7] Forre>st LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, et al. (2005) A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer 92:1834–1836. Article Pubmed
[8] Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, et al. (2012) Infl Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 7:587–594. Article Pubmed
[9] McMillan DC, Scott HR, Watson WS, Preston T, Milroy R, et al. (1998) Longitudinal study of body cell mass depletion and the inflammatory response in cancer patients. Nutr Cancer 31:101–105. Article Pubmed
[10] Miki C, Konishi N, Ojima E, Hatada T, Inoue Y, et al. (2004) C-reactive protein as a prognostic variable that reflects uncontrolled up-regulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig Dis Sci 49:970–976. Article Pubmed
[11] Al-Shaiba R, McMillan DC, Angerson WJ, Leen E, McArdle CS, et al. (2004) The relationship between hypoalbuminaemia, tumour volume and the systemic inflammatory response in patients with colorectal liver metastases. Br J Cancer 91:205–207. Article Pubmed
[12] Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, et al. (2011) Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg 201:186-191. Article Pubmed



