Journal of Vaccines & Immunization
An International Peer-Reviewed Open Access Journal
ISSN 2053-1273

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Vaccines & Immunization
Volume 4, Issue 1, August 2016, Pages 1–8
Original researchOpen Access
Silk-based microneedle transdermal immunization with EV71 vaccines elicits potent immune responses in mice
-
Bingling Xu1,
Waseem K. Raja2,4,
Sangun Lee1,
Weilong Liu3,
Izzuddin M. Diwan2,
Bruce J. Panilaitis2,
David L. Kaplan2 and
Saul Tzipori1,*

- 1 Department of Infectious Disease and Global Health, Tufts University Cummings School of Veterinary Medicine, 200 Westboro Road, North Grafton, MA 01536, USA
- 2 Department of Biomedical Engineering, Science and Technology Center, Tufts University, 4 Colby street, Medford, MA 02155, USA
- 3 Department of Infectious Disease, Shenzhen Third People's Hospital, Guangdong 518020, China
- 4 The Picower Institute for Learning & Memory, Massachusetts Institute of Technology, 43 Vassar St, Cambridge, MA 02139, USA
*Corresponding author: Tzipori S, DVM, PhD, Department of Infectious Disease and Global Health, Tufts University Cummings School of Veterinary Medicine, 200 Westboro Road, North Grafton, MA 01536, USA. Tel.: 508 839-7955; Fax: 508 839-7911; E-mail: Saul.tzipori@tufts.edu
Received 10 May 2016 Revised 7 July 2016 Accepted 18 July 2016 Published 27 July 2016
DOI: http://dx.doi.org/10.14312/2053-1273.2016-1
Copyright: © 2016 Xu B, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Hand, foot, and mouth disease (HFMD), caused by EV71 viral infection, has emerged as a serious public health concern in the Asia-Pacific region. The lack of effective treatment has led to a focus on controlling EV71 epidemics through development of vaccines. This study compares the potency of inactivated whole-virus EV71 vaccine with recombinant viral protein (rVP) subunit vaccine, administered by various immunization routes including intraperitoneal (IP), intradermal (ID), or using a microneedle (MN) patch. The MN patches, made of biocompatible silk proteins loaded with antigens and adjuvant, were applied directly to the skin. Both inactivated virus and rVPs vaccines generated substantial EV71 specific serum IgG, but only the former induced neutralizing antibody. Immunization with silk MN patches induced comparable neutralizing antibody titers to the IP and ID. These findings suggest that inactivated EV71 virus was superior to rVP subunit vaccine in stimulating neutralizing antibody and that with some fine-tuning; the silk-based MN patches are an effective and non-invasive delivery platform for EV71 and possibly for other immunogens.
Keywords: EV71 vaccine; recombinant protein; VP1; VP2; VP3; subunit; transdermal immunization; microneedle; silk
IntroductionTop
Enterovirus 71 (EV71), a human viral pathogen, is a member of the Enterovirus genus of the Picornavirus family and causes blisters and ulcers in the mouth and rashes on the hands and feet, commonly known as human hand, foot, and mouth disease (HFMD). The major target populations are children under five years old, who account for 92.23% of the total cases reported [1]. EV71 can also cause severe clinical symptoms of polio-like paralysis, meningitis, and fatal encephalitis with pulmonary edema and cardiac failure in extreme cases, especially in the age group under one year [1, 2]. Because there are no well-defined parameters to predict the mild, self-limiting form of the disease from the severe form of HFMD, extreme precautions are necessary when administering care to pediatric HFMD patients [3-5]. Over the last decade, large-scale outbreaks of EV71 infection-associated HFMD have occurred in the Asia-Pacific regions [6-9]. The cost of public health care to manage EV71 outbreaks was significant. Since no effective antiviral agents are readily available, developing EV71 vaccines for primary prevention among the very young is considered to be the best control strategy against EV71 [2, 5, 10].
Inactivated whole-virus vaccines are a favorable choice for EV71 vaccine development [11-13]. Due to the demonstrated potent immune responses EV71 stimulates and advanced manufacturing technology, rapid progress has been made towards the licensing of inactivated whole-virus EV71 vaccines [12]. Currently there are five organizations located in Mainland China, Taiwan, and Singapore that have reported clinical trials of inactivated EV71 vaccines [12, 14]. Recombinant subunit vaccines are considered comparatively safer and more cost effective, however, they are generally less immunogenic because of the high purity and lack of conformational molecular structure [13, 15]. Strong adjuvants are therefore usually required and the antigenic properties of various potential subunits should be individually examined [11, 12]. EV71 virions are made of four structural proteins [Virus protein (VP) VP1 (33KDa), VP2 (28KDa), VP3 (27KDa), VP4 (8KDa)]. Capsid proteins VP1, VP2, and VP3 are exposed on the capsid surface, and VP4 is completely buried inside of the capsid [5]. Studies reported that recombinant protein VP1 (rVP1) produced and purified from various expression systems elicited virus-neutralizing antibody responses and protected newborn mice against lethal EV71 challenge, though the results were not consistent [11, 12, 15-20]. There are not many studies which reporting the immunogenicity of recombinant VP2 and VP3 antigens [12].
Classic needle injected vaccination of children present several public safety concerns, especially in the developing countries [21]. Transcutaneous (TC) vaccinations use needle-free devices to topically deliver antigens to the epidermis, making vaccinations non-invasive, less painful for children, more efficient, safer, economical, and highly desirable [13, 22, 23]. Skin is an important peripheral immune organ containing a large network of active antigen presenting cells (APCs) like Langerhans cells and dermal dendritic cells, which take up the antigens and migrate to the proximal lymph nodes where naïve T and B cells will be activated to initiate adaptive immune response [24]. Microneedles are micrometer-scale needles that can be coated with vaccine, creating a transport pathway large enough for delivery of antigens to the targeted skin, but small enough to avoid pain [25]. However, the clinical use of microneedle patches made of silicon, metal, stainless steel, or titanium has faced serious obstacles because microneedles can fracture and remain in the skin, which is a safety issue [26]. Microneedles fabricated with silk protein offer superior mechanical properties and biocompatibility which provides a safer delivery device for vaccination [27]. Furthermore, microneedle patches can be administrated by minimally trained personnel, decreasing the logistical challenges associated with delivery of EV71 vaccines and increasing compliance [14]. Finally, while microneedles could lead to skin infections due to the disruption of this barrier, antibiotics can be stabilized in silk which offers a relatively simple preventative method if needed, while also avoiding systemic exposure [28].
In this study, we evaluated the immunogenicity of inactivated whole-virus EV71 and recombinant subunit EV71 vaccines administered either intraperitoneally (IP), intradermally (ID) or delivered by a silk protein microneedle patch.
Materials and methodsTop
Virus propagation and purification
EV71 strain H (subgenogroup C1; Genbank AY053402) was purchased from American Type Culture Collection (ATCC) (catalog # VR-1432) and used as the inactivated vaccine virus and challenge strain in the in vitro neutralization studies. This strain was originally isolated from the vesicular fluid of a woman with hand-foot and mouth disease, in Wuhan, China in 1987 [29-31].
For viral propagation, a monolayer of 80% confluent Vero cells (ATCC CCL-81) in minimum essential Medium Eagle (Cellgro, Cat. 15-010-CV) containing 2% fetal bovine serum (Gibco, 10082-139) was infected with EV71 virus at a multiplicity of infection of 0.05-0.1. When 90% of the cells displayed the typical enteroviral cytopathic effect (CPE), the virus was harvested and stored in -80°C.
For virus purification, the infected Vero cells were lysed by 3 freeze-thaw cycles to release the virus. Differential centrifugation was applied to first remove the cell debris at 10,000g and then to concentrate the viral particles at 80,000g. Resuspended virus was centrifuged on a 10%∼50% (w/w) continuous sucrose gradient at 80,000g for 4h [11]. Sucrose solution was collected at 20 drops/fraction, and each fraction was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis and western-blot detection of EV71 antigen using mouse anti-VP1 serum, produced in this lab. Fractions rich in EV71 were gathered and filtered through Amicon Ultra-15 Centrifugal Filter Unit (Millipore, UFC905024) to replace the sucrose by sterile phosphate-buffered saline (PBS). EV71 was heat inactivated by 65°C for 30min prior to use as an immunogen and enzyme-linked immunosorbent assay (ELISA) priming protein. The amount of virus protein was quantified using dot-blot by comparing with rVP1 protein of known concentration. Viral titer was determined as the 50% tissue culture infective dose (TCID50) in the Vero cells as described [11].
Recombinant protein rVP1, rVP2 and rVP3 expression and purification
The sequences of VP1, VP2 and VP3 genes were provided by Dr. Weilong Liu according to the sequence of an EV71 clinic strain (Subgenogroup C4) isolated in ShenZhen, China [32]. The rVP1 protein was expressed in Escherichia coli (E. coli) BL21 (DE3) that had been transfected with pET21; VP1 plasmid, which was kindly provided by Dr. Liu [32]. The VP2 and VP3 genes were synthesized de novo and subcloned into expression vectors, and recombinant protein VP2 (rVP2) and recombinant protein VP3 (rVP3) expressed by commercial protein service (GenScript, NJ).
SDS-PAGE, western blot and dot blot analysis
The rVP1, rVP2, and rVP3 proteins and purified virus samples were analyzed by SDS-PAGE and western-blot assays. Purified protein/virus samples were boiled in reducing Laemmli buffer (with 0.4M DTT and 4.8M urea for rVP3 to reduce aggregation), then subjected to SDS-PAGE. For blotting, membranes were blocked with 5% non-fat dry milk dissolved in 0.1% Tween-20 in PBS (noted as PBST) for 1h, washed briefly, and incubated with mouse anti-EV71 serum (1:2000 diluted), followed by goat anti-mouse IgG(H+L)-HRP (Southern Biotech, 1:2000 diluted) as secondary antibody. Membrane was then treated with Amershan™ ECL western blot detect reagent and exposed to autoradiography film for 2-15s to visualize protein bands. Inactivated EV71 virion was semi-quantified by comparing with know-concentration of rVP1 protein using dot blot.
Fabrication of silk microneedle and antigen loading
Silk purification and microneedle fabrication were performed as described previously [27, 28, 33]. Two different groups of antigens were coated onto separate microneedle patches; inactivated EV71 virions, and a subunit mixture (VPmix, an equal mixture of three subunits: rVP1, rVP2, and rVP3). In one group, 30µg of inactivated EV71 virions was mixed with silk solution and 1µg double mutant heat-labile toxin from Escherichia coli (dmLT) as adjuvant. In a second group, 30µg recombinant proteins VPmix were mixed with silk solution and 1µg dmLT were mixed. The antigen solutions were pipetted onto each microneedle patch, then air dried and used within 24 h.
Immunization of animals and sample collection
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Tufts University and performed in compliance with institutional guidelines. Female BALB/c mice at 8-10 weeks old (n = 5 per group) and one New Zealand rabbit were used in immunization studies. One rabbit was immunized subcutaneously with 10µg inactivated EV71 virus with Freud’s complete/incomplete adjuvant four times with two weeks intervals to generate large quantities of anti-EV71 polyclonal antibodies. Groups of mice (n = 5 per group) were vaccinated intraperitoneally four times with two weeks intervals, with 10µg inactivated EV71 whole virus, 10µg recombinant coat protein rVP1, rVP2 or rVP3, or 10µg VPmix (a total of 10µg rVP1, rVP2 and rVP3 equal mixture) in the presence of alum adjuvant (Imject® Alum, Thermo Scientific™). For Alum adsorption, antigen solution was diluted in sterile PBS accordingly and mixed with Imject Alum at final volume ratio of 1:1, and slowly mixed for 30 min at 4°C using lab rotary mixer. For transdermal immunization, mice were first anesthetized with ketamine (80ug/kg of body weight) and Xylazine hydrochloride (8mg/kg of body weight) and hair was shaved from the injection site and then antigens in 20µl solution were injected into two shaved locations in the back (10µl each site). Mice were immunized four times with two weeks intervals. For immunizations using microneedle patches, residual hair was removed by the application of a commercial hair removal cream (Nair; Church & Dwight Co., Inc.) after the shaving. The microneedle patches were applied onto the dried, cleaned skin by gently pressing using the thumb to ensure insertion of the needle tips into the skin. The patches were secured and left on the skin for 48h to deliver the reagent. Animals were immunized with the new patches at the same site bi-weekly four times and blood samples were collected 24h before each immunization and before termination. For each immunization groups, sera samples were collected pre-immunizations and two weeks after each immunization. Mucosal samples (saliva, fecal and vaginal wash) were collected pre-immunization and prior to termination as previously described [34].
ELISA for detection of anti-EV71 antibodies
All animal samples were tested for the presence of EV71 specific IgG, IgG1, IgG2a and IgA antibodies by standard ELISA. Designated priming proteins (0.5µg/ well recombinant proteins, or 1µg /well inactivated EV71 viral particles) were used to coat ELISA plate. Plates were then blocked with 0.1% BSA-PBST for 2h. After washing, diluted samples were applied and incubated for 1hr. After washing, peroxidase-conjugated goat anti-mouse IgG, IgG1, IgG2a or IgA antibody was added as applicable and incubated for 1h. Plates were then washed, and peroxidase substrate (KPL, Cat #50-76-00) was added and allow reactions for 20min before stopping using phosphoric acid (H3PO4). Absorbance values at 450nm were recorded. Absorbance values of the pre-immunized sera were used as reference blanks. The cutoff was defined as average mean of blank plus three times the standard deviation. Samples with absorbance values higher than the cutoff were considered positive. Serum endpoint titers are defined as the highest serum dilution that has a positive read.
In vitro serum neutralization titer assay
Sera were inactivated at 56°C for 30 min before usage. Two-fold diluted sera were mixed with 200 TCID50 or 20 TCID50 of EV71 virus and incubated at 37°C for 2h. After incubation, the serum-virus mixtures were transferred in parallel onto Vero cells (70% confluent) in 96 well plates for infection. Developments of CPE were monitored for 72h. The neutralization titer was defined as the highest serum dilution that protected over 50% of cells from EV71 infection.
Statistics
The statistical analysis was performed with GraphPad Prism 6 software using nonparametric tests. Differences between multiple groups were compared with one way ANOVA test. Value of p<0.05 were considered statistically significant.
ResultsTop
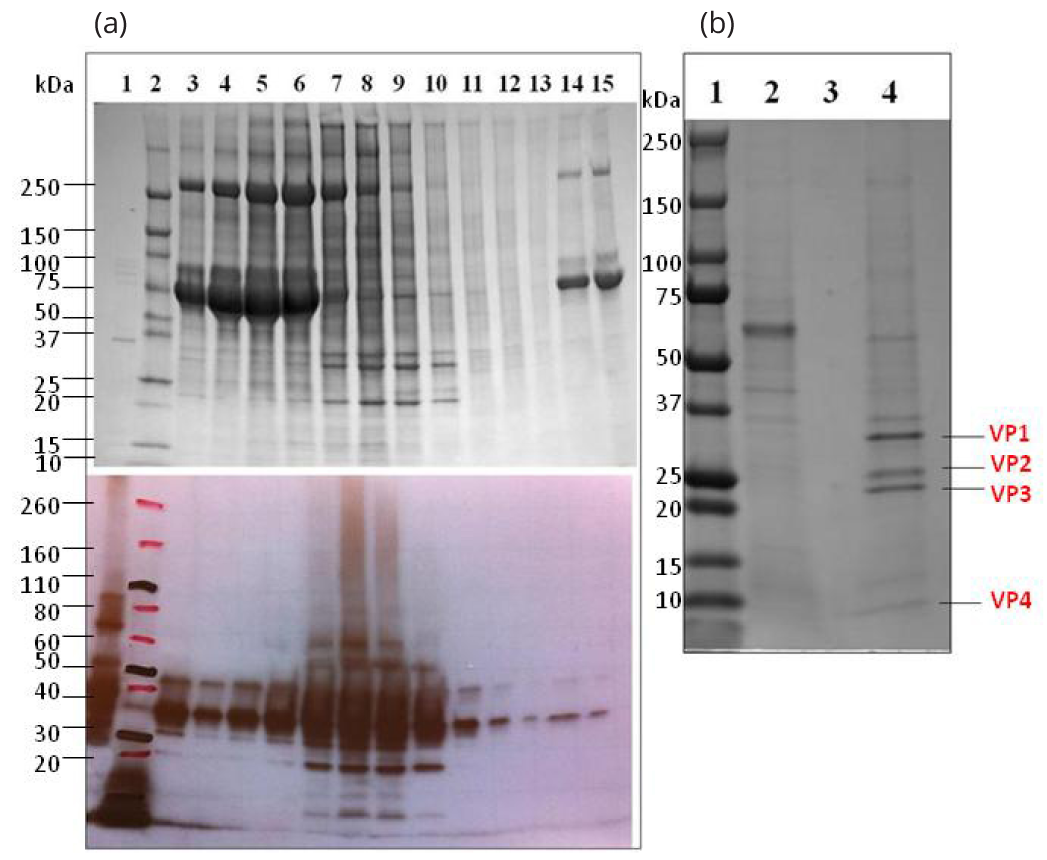
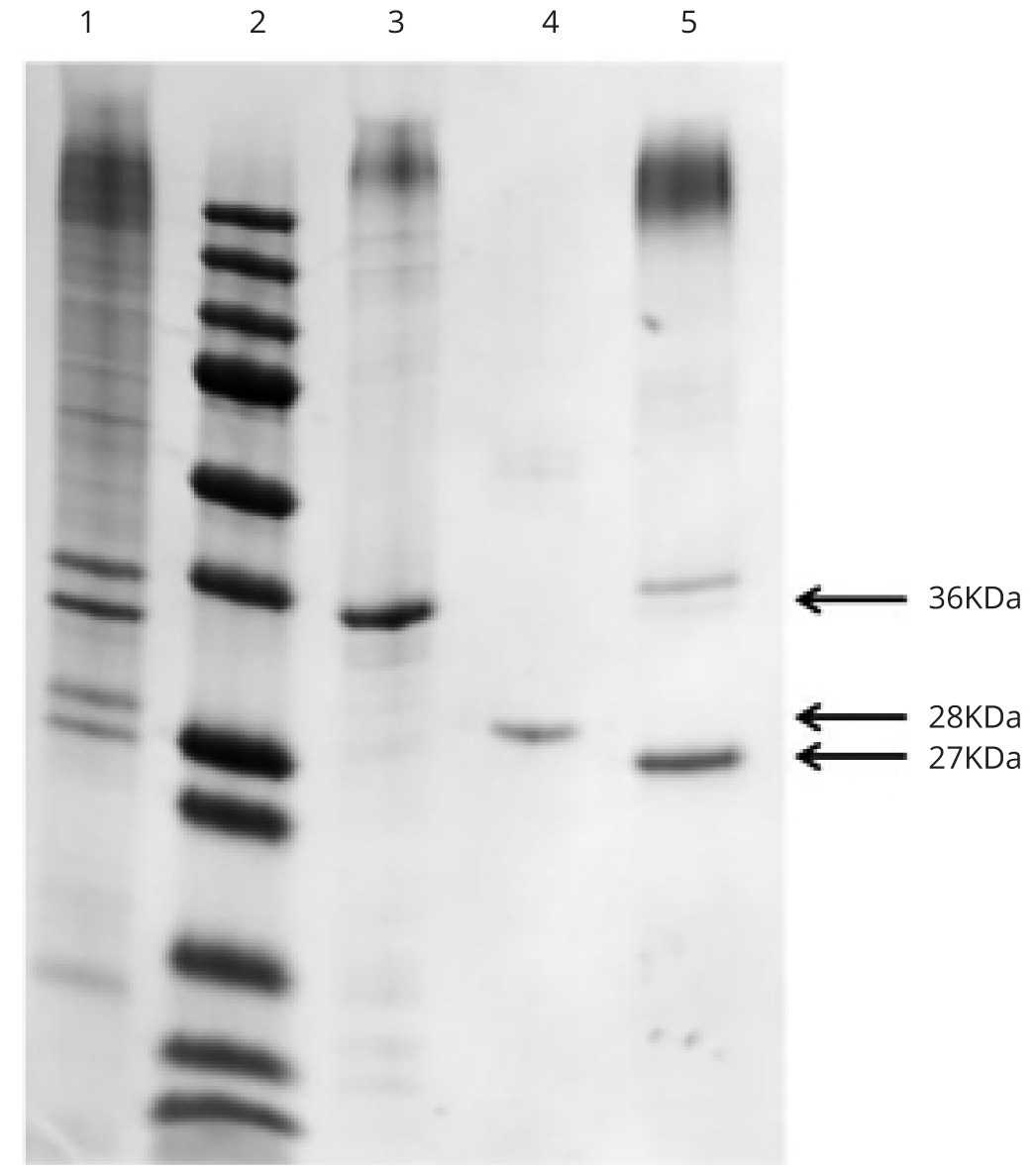
EV71 virus purification and generation of recombinant RVP1, RVP2, and RVP3
Human EV71 strain H was propagated and virus particles were purified (Figure 1a): Capsid proteins VP1 (∼35kDa), VP2 (∼28kDa), VP3 (∼27Kda) and VP4 (∼7.5kDa) of EV71 virions were visualized in SDS-PAGE analysis (Figure 1b). The recombinant proteins rVP1, rVP2 and rVP3, generated in E. coli for use in the EV71 subunit vaccines, were purified and analyzed by SDS-PAGE (Figure 2) and confirmed by western blot (Supplementary Figures 1 and 2).
Immunogenicity study of intraperitoneally immunized inactivated whole-virus and subunit EV71 vaccines
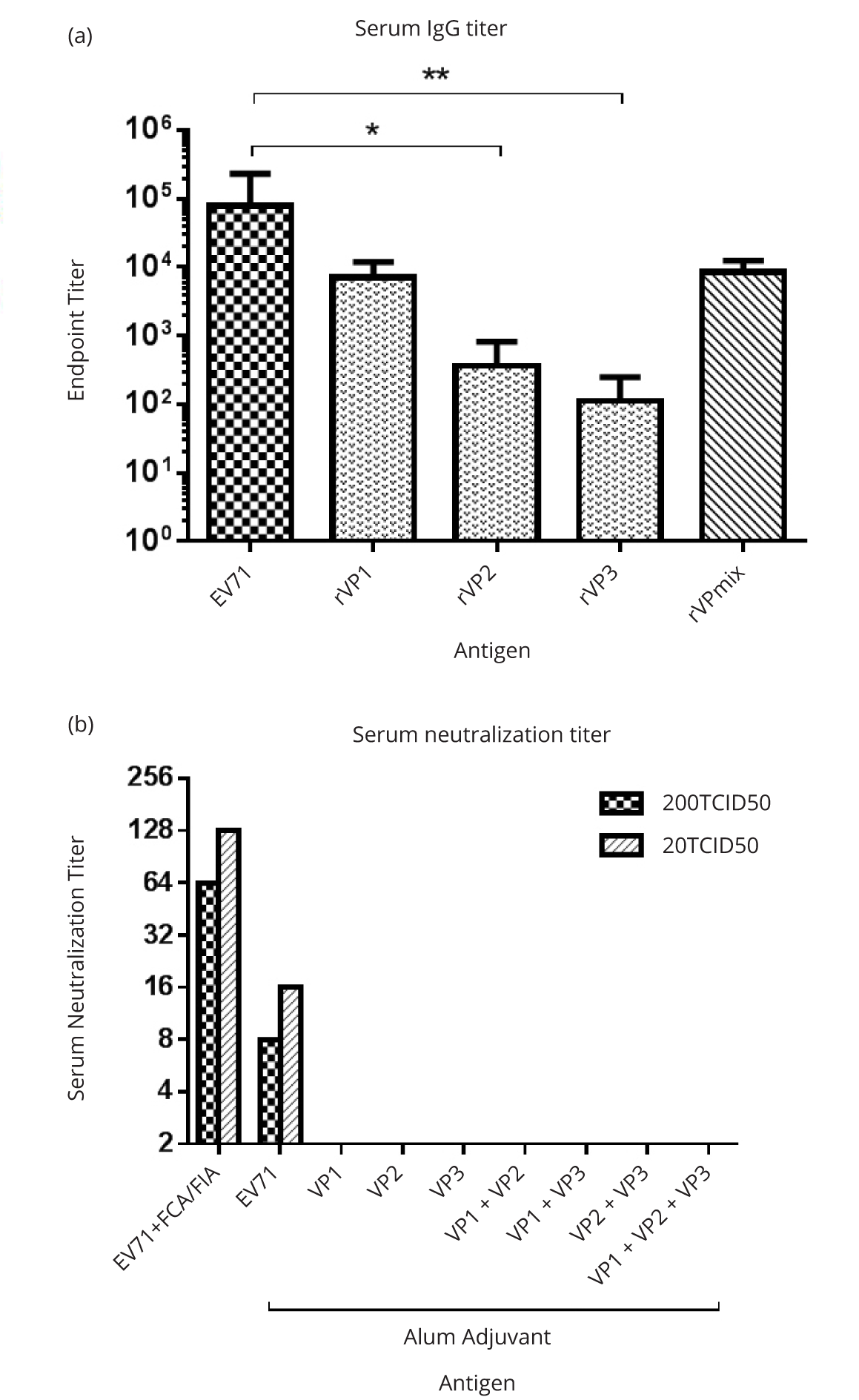
All of the intraperitoneally administered EV71 antigens induced mouse antibody response (Figure 3a). ELISA results showed IgG titer stimulated by inactivated EV71 whole virus was the highest. IgG titers stimulated by a mixture of EV71 subunits rVPmix and by rVP1 alone were comparable, at ten-fold lower than that stimulated by inactivated EV71 (Figure 3a). The IgG stimulated by rVP2 and rVP3 were lower than the anti-EV71 IgG titer by almost 100 fold (P = 0.003) (Figure 3a). Based on the ability to stimulate antibodies, we rank the immunogenicity of tested EV71 antigens in the following order: inactivated EV71 whole virus > rVPmix = rVP1 > rVP2 = rVP3.
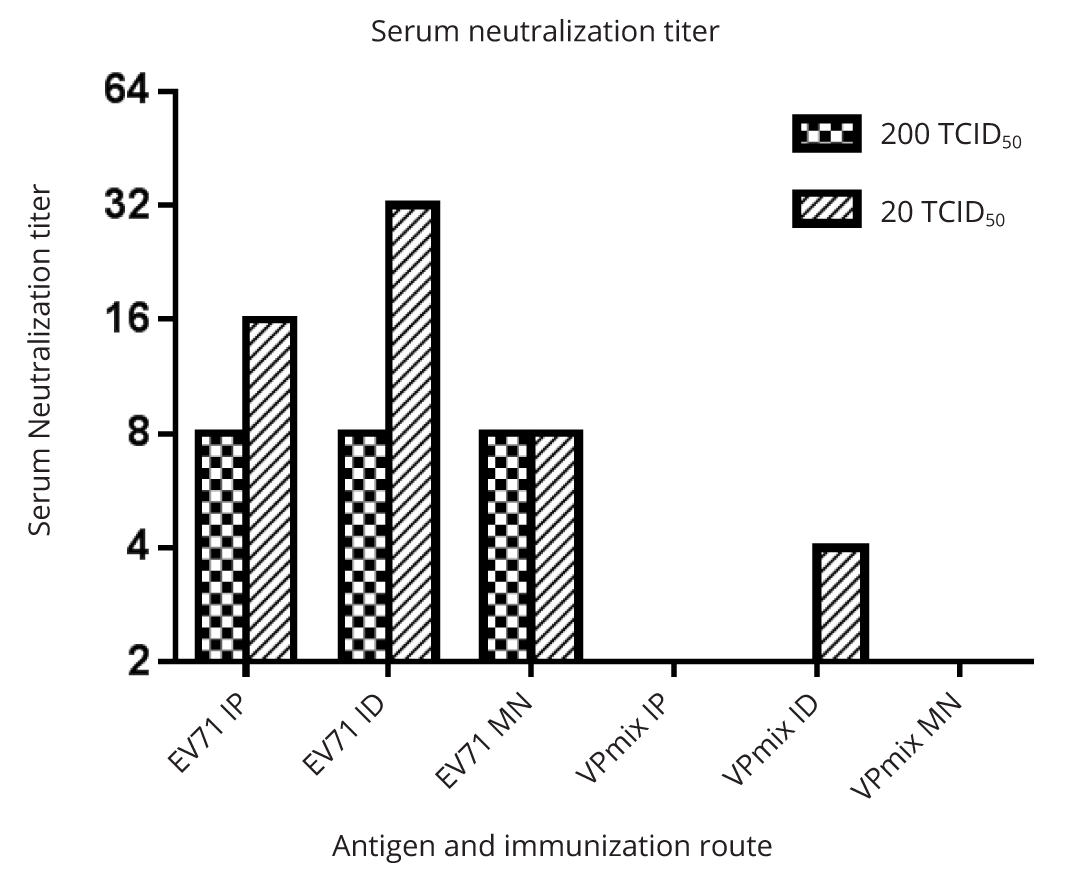
Neutralization assays of the immune sera demonstrated that only the sera from animals (mice and one rabbit) immunized with inactivated whole virus showed a neutralization titer against EV71 infectivity (Figure 3b). The neutralization titers against 20 TCID50 EV71 were 2 fold higher than those against 200 TCID50 EV71 (Figure 3b). Using alum as adjuvant, inactivated EV71 elicited antibody with neutralization titers of 1:16 against 20 TCID50 and 1:8 against 200 TCID50. The rabbit immunized with inactivated EV71 with FCA/FIA adjuvant had higher serum neutralization titers, which were 1:128 against 20 TCID50 and 1:64 against 200 TCID50 of EV71. Sera from mice immunized with either individual recombinant EV71 subunit or combinations thereof failed to neutralize EV71 infectivity in vitro (Figure 3b), despite of their high antibody titers (Figure 3a).
Silk microneedle patch immunization induces neutralizing immune responses
We investigated whether EV71 antigen-coated, silk-based microneedles, induced EV71-specific immune responses. Based on experience, microneedle delivery is less efficient compared to injectable route deliveries. For this reason, we loaded three times more antigen on microneedle patch in order to induce a immune response comparable to that induced by injectable route. However, for inactivated whole-virus EV71 vaccine, microneedle skin patches stimulated an anti-EV71 IgG titer of 103 in the sera, which was 10 to 100 fold lower (p = 0.045) than that stimulated by IP and ID delivered by injections (Figure 4a). IP and ID deliveries stimulated measurable serum IgG after the 1st immunization, whereas microneedle delivery stimulated measurable IgG only after 2nd immunization (Figure 4a). We also evaluated the subunits rVPmix vaccination and found the results to be very similar to those obtained from the inactivated EV71 whole virus: Microneedle immunization stimulated an anti-rVPmix IgG titer of 104, which tended to be lower (p = 0.018) than those from IP and ID immunizations by 10 to 100 fold (Figure 4b).
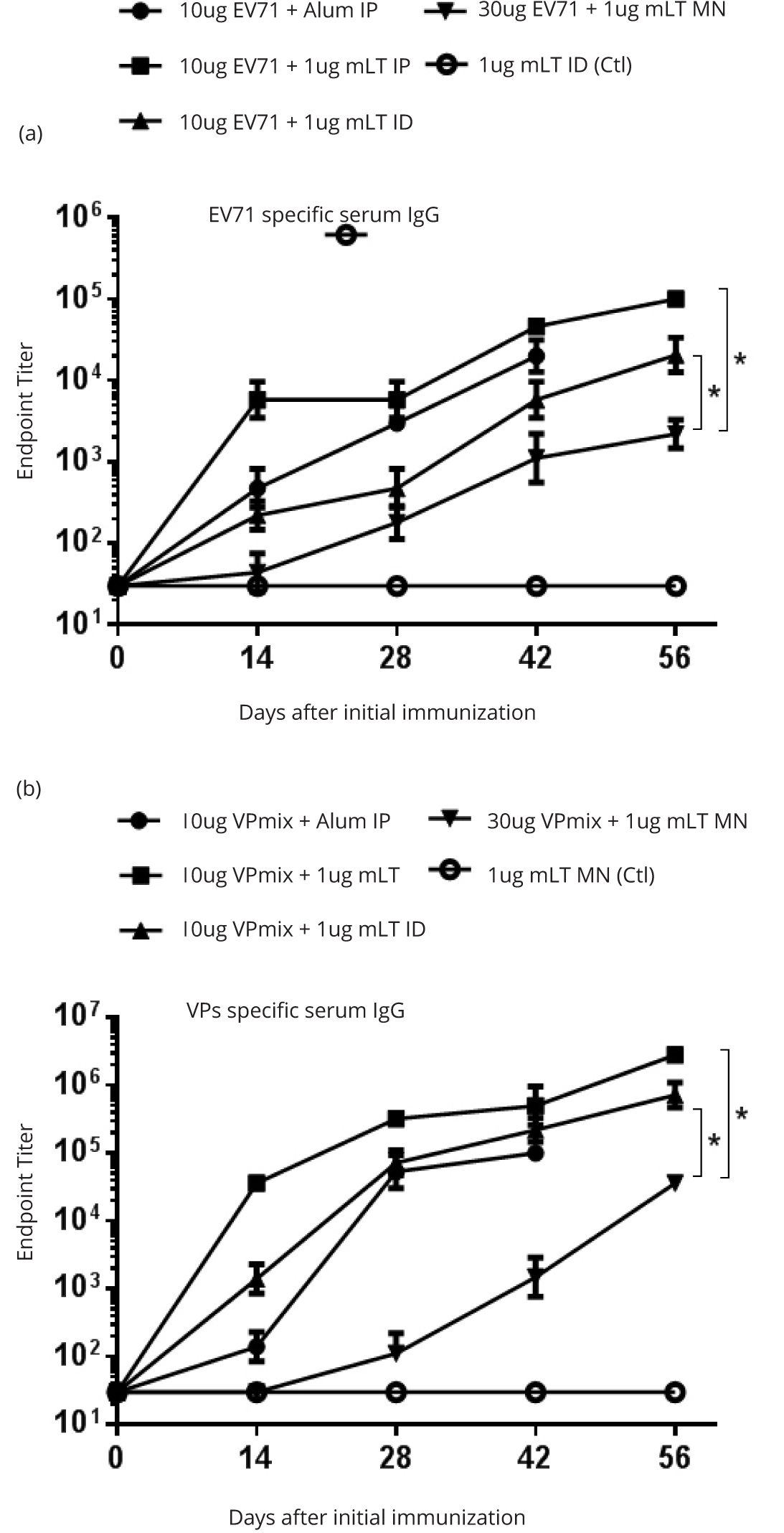
Sera induced by rVPmix via either microneedle, ID or IP administration showed no neutralizing activity, except that ID immunization induced a marginal neutralizing titer of 1:4 against 20 TCID50 EV71 infection (Figure 5). In contrast, all of the sera induced by inactivated EV71 whole virus showed serum neutralizing titers. Sera induced by inactivated EV71 loaded microneedles had a neutralizing titer of 1:8 against 20 TCID50 and 200 TCID50 of EV71. Comparing to microneedle delivery, ID and IP injections stimulated higher neutralization titers against 20 TCID50, which were 1:32 and 1:16, respectively (Figure 5). These results were consistent with the results obtained from the immunogenicity study of intraperitoneal immunization (Figure 3b).
Serum IgG1:IgG2a ratio induced by microneedle patch immunization
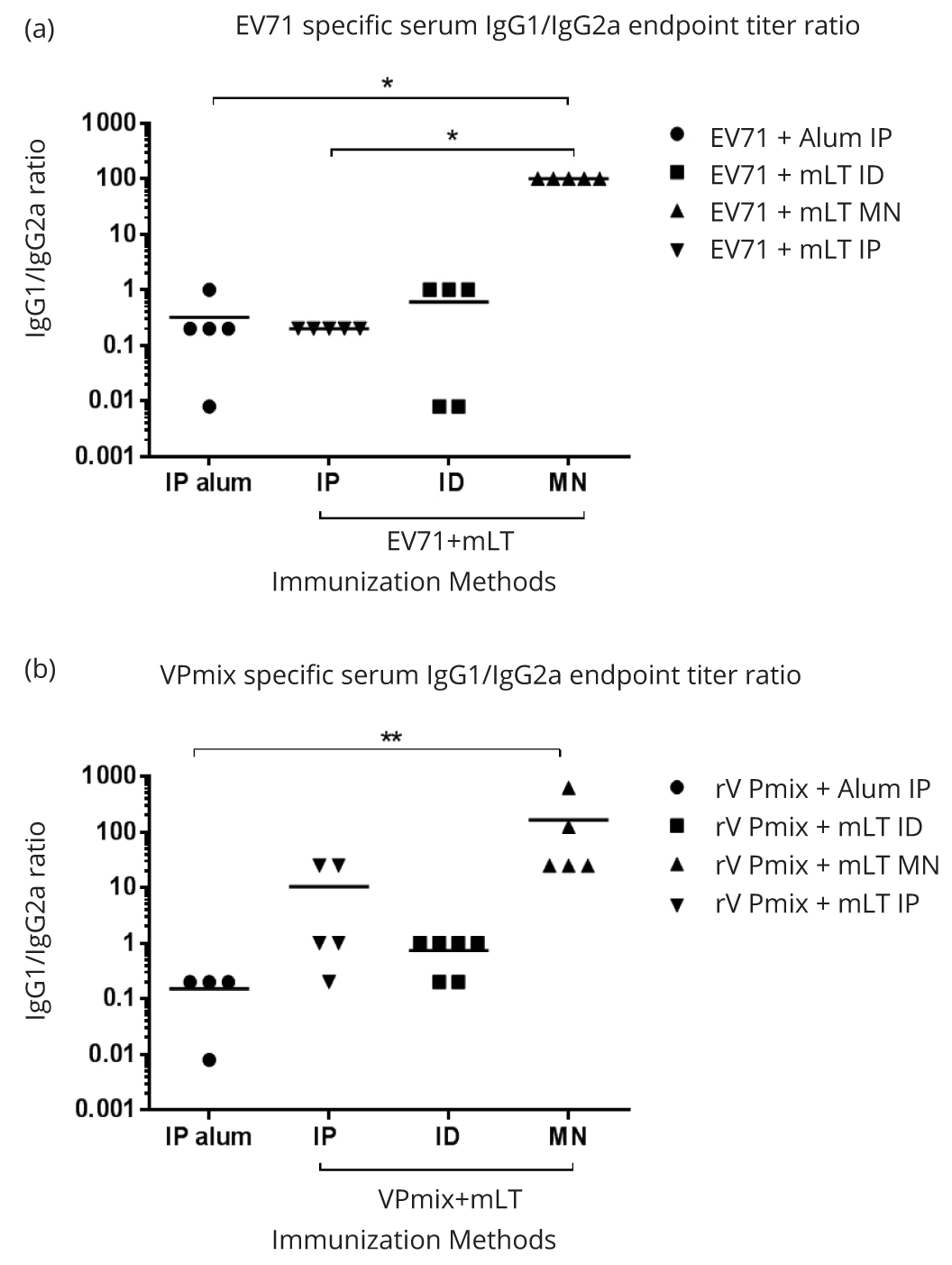
To evaluate the Th1/Th2 balance induced by transcutaneous immunizations, we analyzed the antigen specific IgG1 and IgG2a titers using ELISA. In the inactivated EV71 vaccination groups, microneedle stimulated a dominant Th2-type IgG1 subclass over Th1-type IgG2a subclass, with the IgG1/IgG2a ratio of 100:1 (Figure 6a). ID and IP immunizations with the same antigen and adjuvant induced a balanced expression of EV71 specific IgG1 and IgG2a at the ratio of almost 1 (Figure 6a). IP injection using inactivated EV71 and alum adjuvants also induced balanced EV71 specific IgG1 and IgG2a (Figure 6a). Similarly, in the rVPmix immunization groups, microneedle induced a dominant IgG1 subclass, with an IgG1:IgG2a ratio of 100:1 (Figure 6b). ID immunization with rVPmix and mLT, and IP immunization with rVPmix and alum, both induced balanced IgG1/IgG2a ratio of almost 1. Interestingly, IP immunization with rVPmix and mLT induced a slightly Th2 biased IgG1/IgG2a ratio of 10 (Figure 6b).
Mucosal antibodies induced by microneedle patch immunization
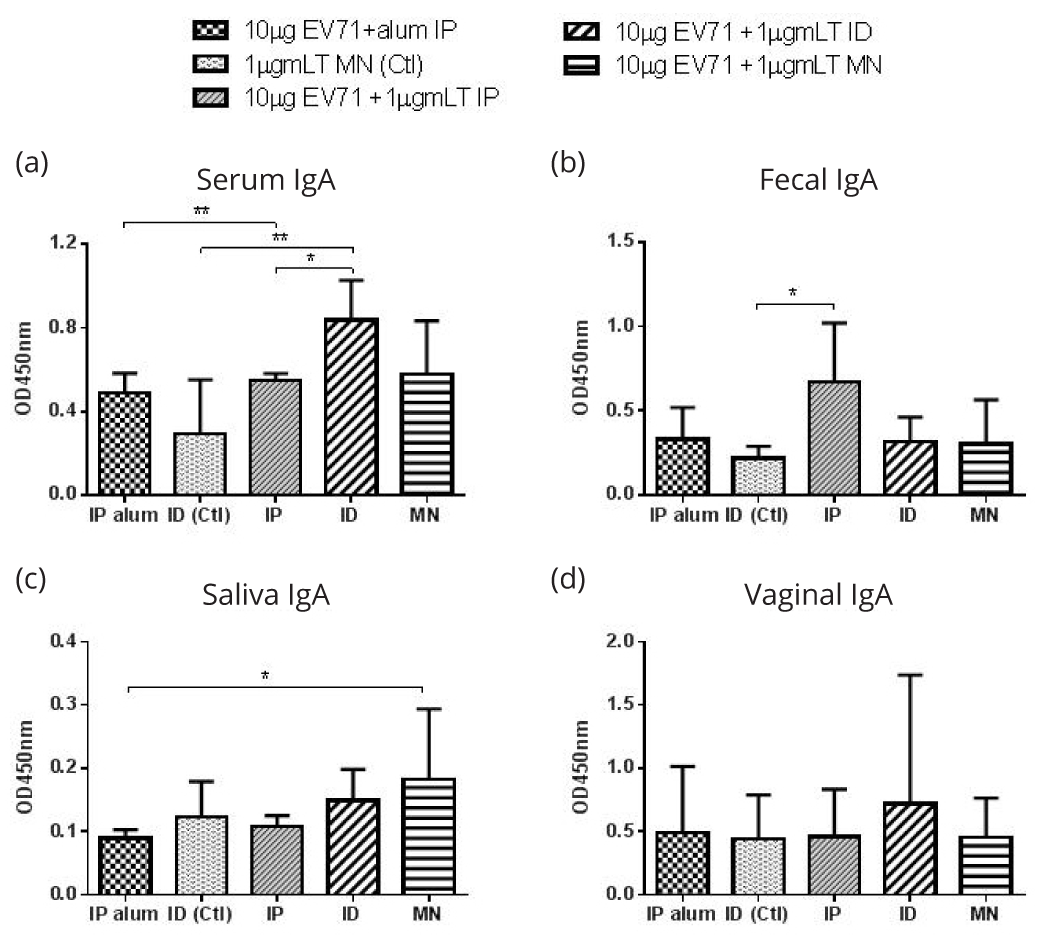
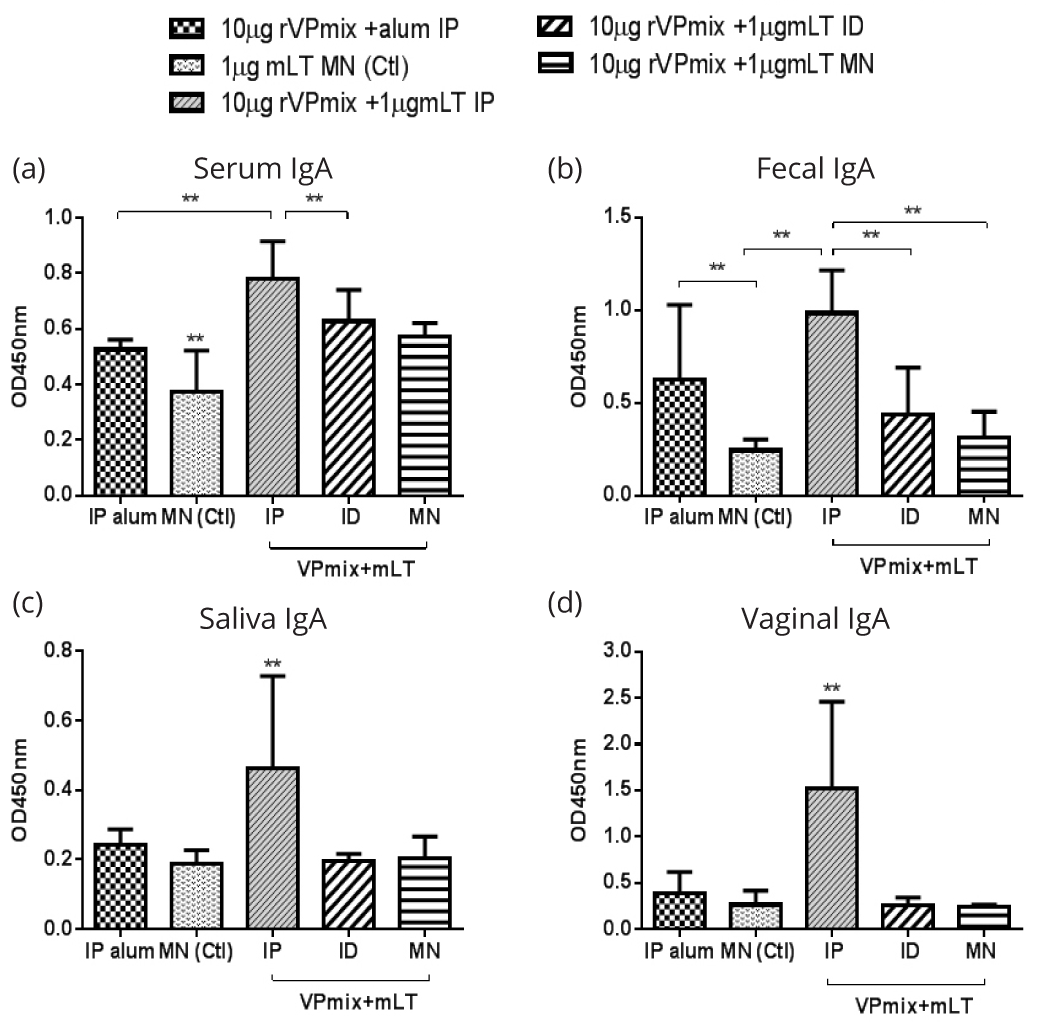
To evaluate the mucosal immune response, we collected mucosal secretion samples (saliva, feces, and vaginal wash) and measured the antigen specific secretory antibody IgA. In the inactivated EV71 immunization groups, ID immunization stimulated significantly higher serum anti-EV71 IgA than the IP immunizations (Figure 7a). For fecal IgA, IP immunization with inactivated EV71 and mLT stimulated higher anti-EV71 IgA level than control (mLT only) group (Figure 7b). In saliva, microneedle delivery stimulated the highest IgA, and the anti-EV71 IgA of other groups was similar to that of the control (Figure 7c). For vaginal samples, none of the groups generated significant IgA levels (Figure 7d). For rVPmix vaccinated groups, IP immunization using rVPmix with mLT stimulated higher anti-rVPmix IgA levels compared to all other immunization methods in serum, saliva, fecal, and vaginal samples (Figure 8a-d).
DiscussionTop
Comparing the potency of inactivated EV71 virus with the three subunit viral proteins, individually or mixed, demonstrated that inactivated EV71 virus was superior and essential in inducing neutralizing antibodies. This result is consistent with previous reports that rVP1 with alum were not able to induce neutralizing antibody response against EV71 [11]. These results may suggest the importance of conformational epitopes, which are absent in recombinant subunit but present in inactivated EV71 virions, playing an essential role in inducing neutralizing antibody against EV71 infectivity.
Microneedles loaded with inactivated EV71 virus and recombinant EV71 subunits both induced EV71 specific antibodies, suggesting that this system is not only applicable to soluble antigens like recombinant proteins, but also to particulate antigens. However, antibody titers induced by microneedle immunization were lower than those of ID injection, which are comparable to IP immunization. This result demonstrated that although microneedles were able to deliver antigens into the epidermis and dermis where antigen presenting cells reside, the delivery efficiency is inferior to ID injections. This could be because portion of antigens were lost crossing the stratum corneum barrier during the patch application process, which calls for additional characterization of delivery kinetics. Another possible contributing factor is that the microneedle length of 750nm was not optimized for the mouse skin anatomy, in which the antigens were not delivered to the skin layer richest in APCs. While the utility of the non-invasive microneedle patch as a method of vaccine delivery appears to be less effective than ID, the methodology is in its infancy, and with further fine-tuning this application has the potential of dramatically altering the current invasive methods of delivery, particularly in infants and children.
IgG1 and IgG2a antibody subclasses, markers of Th2 and Th1 immune responses, respectively, showed that microneedle immunization with inactivated EV71 whole virus and recombinant subunit proteins with mLT adjuvant stimulated predominantly Th2 responses as compared to the other groups. The ID injection, however, stimulated a balanced IgG1/IgG2a ratio of almost 1. This distinct difference between ID injection and microneedles may indicate differences in the APC subsets involved in antigen capture [26]. The APC subsets in each layer of the skin are different, such as LCs residing in epidermis and dDCs in the dermis [24, 35-37]. In the case of EV71 vaccine, our goal is to generate high level of mucosal and systemic neutralizing antibody, and to prevent the development of viraemia. In this sense, a strong Th2 immune response is theoretically desirable. However, the precise correlate/immune marker of protection of EV71 infection is not established yet. Further clinical research is needed to establish the correlates of EV71 vaccine-induced immunity in human, which would serve as a guideline to preclinical EV71 vaccine development.
Some reports show transcutaneous immunization with adjuvants like cholera toxin, E. coli heat-labile enterotoxin and CpG oligodeoxynucleotide could induce a mucosal response, increase IgA production, and reduce the amount of antigen needed [36-38]. We therefore included the adjuvant mLT in this study. Unfortunately, neither our microneedle nor ID immunization induced higher antigen specific IgA than the base line control group, which was also reported by Matsuo et al. [23]. The only exception is that rVPmix and mLT immunization via ID injection induced higher anti-rVPmix serum IgA than that of mLT only controls (Figure 7 and 8). We will test in a future study if microneedle delivery of EV71 antigens with different adjuvants induces a better immune response compared to that with mLT.
ConclusionTop
The objective of this study was to evaluate transcutaneous EV71 vaccination using silk protein-based microneedle patches and characterize the induced immune responses. Our study demonstrated that silk microneedle safely and effectively induced immune responses against soluble and particulate EV71 antigens in mice. Microneedle immunization features advantages including inexpensive manufacturing, small size for easy storage and distribution, a simple administration process that enables self-vaccination, and likely better acceptance by the general public due to the reduction in pain when compared to injection-based delivery. With fine tuning of this immunization strategy, this could pave the way for development of a safe, effective, non-invasive and low cost EV71 vaccine.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
I would like to extend a special thank to Dr. Jennifer Steele and Dr. Hellen Amuguni for helping to edit this manuscript.
Funding
This work was funded by Tufts Collaborates grant [V340103]; the China Scholarship Council; and the Defense Threat Reduction Agency (DTRA).
Supplementary dataTop
ReferencesTop
[1]Chang ZR, Zhang J, Sun JL, Zhang WD, Wang ZJ. Epidemiological features of hand, foot and mouth disease in China, 2008 - 2009. Zhonghua liu xing bing xue za zhi. 2011; 32(7):676–680.Article Pubmed
[2]Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010; 9(11):1097–1105.Article Pubmed
[3]Li W, Teng G, Tong H, Jiao Y, Zhang T, et al. Study on risk factors for severe hand, foot and mouth disease in China. PLoS One. 2014; 9(1):e87603.Article Pubmed
[4]Fang Y, Wang S, Zhang L, Guo Z, Huang Z, et al. Risk factors of severe hand, foot and mouth disease: A meta-analysis. Scand J Infect Dis. 2014; 46(7):515–522.Article Pubmed
[5]Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010; 10(11):778–790.Article Pubmed
[6]Li Y, Zhang J, Zhang X. Modeling and preventive measures of hand, foot and mouth disease (HFMD) in China. Int J Environ Res Public Health. 2014; 11(3):3108–3117.Article Pubmed
[7]Xing W, Liao Q, Viboud C, Zhang J, Sun J, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. 2014; 14(4):308–318.Article Pubmed
[8]McMinn PC. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr Opin Virol. 2012; 2(2):199–205.Article Pubmed
[9]Sun LM, Zheng HY, Zheng HZ, Guo X, He JF, et al. An enterovirus 71 epidemic in Guangdong Province of China, 2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis. 2011; 64(1):13–18.Article Pubmed
[10]Wang SM, Liu CC. Enterovirus 71: epidemiology, pathogenesis and management. Expert Rev Anti Infect Ther. 2009; 7(6):735–742.Article Pubmed
[11]Wu CN, Lin YC, Fann C, Liao NS, Shih SR, et al. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine. 2001; 20(5–6):895–904.Article Pubmed
[12]Chou AH, Liu CC, Chang JY, Lien SP, Guo MS, et al. Immunological evaluation and comparison of different EV71 vaccine candidates. Clin Dev Immunol. 2012; 2012:831282.Article Pubmed
[13]Levine MM. "IDEAL" vaccines for resource poor settings. Vaccine. 2011; 29 Suppl 4:D116–125.Article Pubmed
[14]Chong P, Liu CC, Chow YH, Chou AH, Klein M. Review of enterovirus 71 vacines. Clin Infect Dis. 2015; 60(5):797–803.Article Pubmed
[15]Chong P, Guo MS, Lin FH, Hsiao KN, Weng SY, et al. Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLoS One. 2012; 7(11):e49973.Article Pubmed
[16]Ch'ng WC, Stanbridge EJ, Wong KT, Ong KC, Yusoff K, et al. Immunization with recombinant enterovirus 71 viral capsid protein 1 fragment stimulated antibody responses in hamsters. Virol J. 2012; 9:155.Article Pubmed
[17]Wang M, Jiang S, Wang Y. Recombinant VP1 protein expressed in Pichia pastoris induces protective immune responses against EV71 in mice. Biochem Biophys Res Commun. 2013; 430(1):387–393.Article Pubmed
[18]Ku Z, Shi J, Liu Q, Huang Z. Development of murine monoclonal antibodies with potent neutralization effects on enterovirus 71. J Virol Methods. 2012; 186(1–2):193–197.Article Pubmed
[19]Tian X, Su X, Li X, Li H, Li T, et al. Protection against enterovirus 71 with neutralizing epitope incorporation within adenovirus type 3 hexon. PLoS One. 2012; 7(7):e41381.Article Pubmed
[20]Foo DG, Alonso S, Chow VT, Poh CL. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect. 2007; 9(11):1299–1306.Article Pubmed
[21]Michelle Kermode. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot. Int. 2004; 19(1):95–103.Article Pubmed
[22]Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine. 2013; 31(34):3403–3409.Article Pubmed
[23]Matsuo K, Hirobe S, Yokota Y, Ayabe Y, Seto M, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. J Control Release. 2012; 160(3):495–501.Article Pubmed
[24]Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012; 2:357.Article Pubmed
[25]Ryan F. Donnelly, Thakur Raghu Raj Singh, A. David Woolfson. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010 May; 17(4):187–207.Article Pubmed
[26]Matsuo K, Yokota Y, Zhai Y, Quan YS, Kamiyama F, et al. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J Control Release. 2012; 161(1):10–17.Article Pubmed
[27]Raja WK, Maccorkle S, Diwan IM, Abdurrob A, Lu J, et al. Transdermal Delivery Devices: Fabrication, Mechanics and Drug Release from Silk. Small. 2013. 9(21):3704–3713.Article Pubmed
[28]Konstantino tsioris WKR, Pritchard EM, Panilatis B, Kaplan DL, Omenetto FG. Fabrication of silk microneedles for controlled-release drug delivery. Adv Funct Mater. 2012; (22):330–335.Article
[29]ZM Z. First isolation of enterovirus type 71 from vesicle fluid of an adult patient with hand-foot-mouth disease in China. Virologica Sinica. 1989; 4:375–382.
[30]Zheng ZM, Bankowski MJ, He PJ, Caueffield D, Neumann MA, et al. Comparison of enterovirus 71 (E71) isolated from a patient with hand-foot-and-mouth disease in China to prototype E71 BrCr strain by polymerase chain reaction using a unique primer pair. Clin Diagn Virol. 1993; 1(2):137–139.Article Pubmed
[31]Zheng ZM, He PJ, Caueffield D, Neumann M, Specter S, et al. Enterovirus 71 isolated from China is serologically similar to the prototype E71 BrCr strain but differs in the 5'-noncoding region. J Med Virol. 1995; 47(2):161–167.Article Pubmed
[32]Xie JJ, Yang GL, Liu YX, Liu WL, Chen XC, et al. Development of a serological ELISA kit for detection of EV71 infection associated with hand-foot and mouth disease and its clinical application. Chinese Journal of Laboratory Medicine. 2009; 32(11):1262–1265.Article
[33]Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011; 6(10):1612–1631.Article Pubmed
[34]Amuguni JH, Lee S, Kerstein KO, Brown DW, Belitsky BR, et al. Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice. Vaccine. 2011; 29(29–30):4778–4784.Article Pubmed
[35]Hiraishi Y, Nakagawa T, Quan YS, Kamiyama F, Hirobe S, et al. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. International journal of pharmaceutics, 2013. 441(1–2):570–579.Article Pubmed
[36]Quan FS, Kim YC, Song JM, Hwang HS, Compans RW, et al. Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch. Clin Vaccine Immunol. 2013; 20(9):1433–1439.Article Pubmed
[37]Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, at al. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013; 31(34):3396–3402.Article Pubmed
[38]Lawson LB, Clements JD, Freytag LC. Mucosal immune responses induced by transcutaneous vaccines. Curr Top Microbiol Immunol. 2012; 354:19–37.Article Pubmed


