Journal of Clinical Pharmacology and Toxicology
An International Peer-Reviewed Open Access Journal


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Clinical Pharmacology and Toxicology
Volume 1, Issue 2, November 2016, Pages 8–11
Original researchOpen Access
Antithrombotic activity of SBT-828 in models of arterial thrombosis
- 1 Department of Pharmacology, Volgograd State Medical University, 1, Pavshikh Bortsov Sq., Volgograd, 400131, Russia
- 2 Department of Chemistry, Southern Federal University-105/42 Bolshaya Sadovaya Str., Rostov-on-Don, 344006, Russia
*Corresponding author: Aida Fatikhovna Kucheryavenko, M.D., Associate professor, Department of Pharmacology, Volgograd State medical University, Volgograd, Russia. Tel.: +7 902 364 02 03; E-mail: aidakucheryavenko@yandex.ru
Received 1 July 2015 Revised 30 September 2016 Accepted 10 October 2016 Published 24 October 2016
Copyright: © 2016 Kucheryavenko AF, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
A comparative study of antithrombotic activity of SBT-828, a novel indole derivative, which showed potent antiaggregant activity in vitro, and aspirin, was performed on a murine model of systemic epinephrine-collagen arterial thrombosis, as well as on a murine model of electrically-induced carotid artery thrombosis. The indole derivative was three times greater than the comparator in its ability to prevent mortality of white outbred mice. SBT-828 had a dose-dependent antithrombotic activity in an electrically-induced thrombosis model and showed superiority over the comparator (aspirin) by 1.8 times.
Keywords: platelet aggregation; antithrombotic activity; indole; SBT-828; aspirin
IntroductionTop
Platelets are known to be key mediators of thrombosis [1, 2]. The study of red blood cells has identified a key role of platelet aggregation inhibitors in the treatment and prevention of thrombosis and has resulted in further studies of both new and well-known antiaggregants, which demonstrated their effectiveness on a large scale [3, 4]. Aspirin, which inhibits COX pathway in platelet aggregation as well as adenosine diphosphate receptor antagonists acting at the purinerdic receptors of P2Y12 - ticlopidine, clopidogrel, prasugrel, ticangrelor, cangrelor, is most commonly used in clinical practice [5-7]. However, some of these drugs have lot of drawbacks, such as resistance, risk of neutropenia, gastrointestinal bleeding and thrombocytopenia. Therefore, to develop more safe and effective antiplatelet agents may be a promising approach for prevention and treatment of atherothrombosis [8-10].
In our previous studies, we identified a potent antiaggregant activity of a novel indole derivative in vitro and discovered a promising SBT-828 compound. In this relation, antithrombotic effects of the compound were evaluated on various arterial thrombosis models.
Materials and methodsTop
A comparative experimental study of antithrombotic activity of SBT-828 [11] novel indole derivative and aspirin (Sigma, USA) was conducted. The experiments were performed using 55 male white nonlinear rats with body weights of 300-350 g, 60 white outbred male and female mice with body weight of 20-32g, which were kept in the vivarium at 22-24°C, relative air humidity 40-50% and natural light. The animals received a standard diet (Federal/state standard/GOST R 50258-92). All the animals used for the experiment were healthy with no change in their behavior, with no sleep-wake schedule disorders or change in food appetite. 24 h prior to the experiments, the animals were deprived of food; however, they had free access to water. The experimental protocol was approved by the local ethics committee (Protocol No. 154-2012). All the experiments were conducted according to the guidelines and regulations of animal experiments (GOST 51000.3-96 and 51000.4-96; European convention for the protection of vertebrate animals used for experimental and other scientific purposes (1997); Good laboratory practice guidelines in Russian Federation No. 267 of June, 19, 2003).
The acute toxicity
The acute toxicity was determined on not inbred white male mice weighing 20-25 g by intraperitoneal injection. All animals were observed for two weeks after injection. To calculate the value of toxicological data LD50, Lichfild-Vilkokson’s method was used in accordance with the requirements and regulations of the Federal Service on Surveillance in healthcare and social development (Mironov AN, 2012).
Collagen/epinephrine-induced model of thrombosis in mice
A cellular thrombosis model was created according to a certain technique [12]. A mixture of collagen solution at a dose of 0.5 mg/kg (Sigma, USA) and adrenaline solution at a dose of 0.06 mg/kg (Sigma, USA) was used to develop a thrombotic agent, 0.1 ml of this mixture was injected over 10 seconds into the caudal vein of the animals. Aspirin at a dose of 20 mg/kg (Sigma, USA) was administered as a comparator. The test compound at a dose similar to the dose of the comparator and amounted to 47 mg/kg was administered 2 h before modeling thrombosis. An equivalent volume of solvent was injected to the control animals. The effectiveness of test compounds was measured as, to the number of animals able to survive compared to the controls and as to the amount of thrombi in the lungs. The surviving animals were monitored for 24 h. The mice were killed following ether anesthesia. Dead mice were autopsied, and histological examination of the autopsied lungs was performed. Histological examination of the liver, heart, kidneys and brain was also performed in the control animals. The samples were fixed in 10% neutral buffered formalin (pH 7.4) for 24 h. They were then desiccated and embedded in paraffin wax. Microtomic sections (4-6 μm wide) were made from paraffin blocks and were stained with ematoxylin-eosin [13]. The samples were photographed using a Micros (Austria) microscope and Olympus digital camera (Japan, 4.0 MegaPixels) equipped with the ×10, ×40 objectives and ×10 ocular.
Electrically-induced thrombosis model
An electrically-induced arterial thrombosis model was created in rats using a certain technique [14]. Carotid artery thrombosis was experimentally induced by the electric current passing through the anode. For this purpose, chloral hydrate (Organica, Russia) at a dose of 400 mg/kg was administered to the animals 2 h following the intake of the test compounds. The compounds were dissolved in 2 ml of distilled water. An equivalent amount of solvent was given to the control animals. Carotid artery thrombosis was induced by permanent electric current of 12 V and 50 mA. Electric current was used until complete occlusion of the vessel. Arterial blood flow was recorded using Minimaks-Doppler-K, Doppler ultrasound machine (Minimaks, St. Petersburg, Russia). The blood flow was recorded until total occlusion of carotid artery which is characterized by an absence of pulse in the carotid artery above the area of application of a thrombotic agent as well as by the specific arterial sound. SBT-828 compound was injected at a dose of 23, 35 and 47 mg/kg, while the comparator was given a dose of 20, 60 and 125 mg/kg. The effectiveness of test compounds was measured as prolongation of time interval needed for carotid artery total occlusion. An ED50 of the test compounds was defined as the dose of the compound at which, the duration of thrombus formation is increased by 50%. Aspirin was used as a comparator.
Statistics
Fischer exact test was used to perform statistical confidence analysis of antithrombotic effects (that is defining the proportion of animals which were able to survive in both the experimental and control groups). Morphological investigation was carried out to evaluate the presence of thrombus in arterial vascular walls of muscular arteries. The obtained findings were processed using Statistica/w5.0. Basic statistical analysis software for Windows (Stat.Soft. Inc., USA). «VideoTestMorpho-4» software was used to perform statistical analysis of histological findings. ED50 was calculated using Microsoft Excell regression equation, 2003. Statistical analysis of experimental findings was carried out using Mann-Whitney test and Microsoft Excell statistical software package, 2006.
Results and discussionTop
The value of LD50 for the substance SBT-828 was - 220 mg/kg. From the obtained LD50 value, the substance can be classified as low toxic substance in accordance with IV classification of Sanotsky (1975).
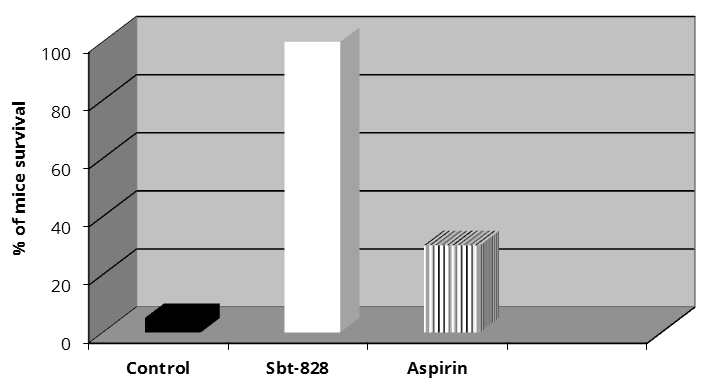
Death of 95% of mice (similar findings were recorded in other reports) was observed in a cellular thrombosis model of the control group [15, 16]. The following well-pronounced features of respiratory impairment were recorded: increased respiration rate and shallow breathing; ophthalmocele and iris colour changes. We also observed lower extremity paresis. The mice did not respond to any impulses and were unable to draw away their rear legs on pressing them hard. Intermittent cramps were visualized and the animals had a specific body posture in which their rear legs were straightened and retracted. The animals died within 1-3 min after the administration of the thrombotic agent. SBT-828 administered at an adjusted dose, prevented death in 100% of animals whose motor activity restored within 1-1.5 min. The proportion of surviving mice which had received aspirin was 30% (Figure 1).
Literature review [12] shows that mortality of animals in an experimental collagen-adrenalin-induced thrombosis model was caused by massive lung microvascular occlusion by thrombolytic agents. However, to prove this fact, histological examination of the animal liver, heart, kidneys and brain, in addition to that of the lungs, had to be carried out. Experimental findings showed that microvessels within these organs remained intact. This information was also confirmed by similar findings in other studies [17].
A mixture of collagen solution at a dose of 0.5 mg/kg and epinephrine solution at a dose of 0.06 mg/kg was used to develop a thrombotic agent. The test compounds were administered 2 h before the modeling of thrombosis. Solvent was injected to the control animals (n = 20 in all groups). In the control group, only 5% of mice had survived. In the group where SBT-828 was administered in dose of 47 mg/kg, 100% survival of the mice was observed. Аspirin administered in dose of 20 mg/kg revealed survival of 70% of mice.
Microscopic investigation of the lung tissue samples in the control group revealed predominant medium-sized alveoli. White microthrombi adhering to the vascular wall in the arterial lumen were found in a large number of vessels of the microcirculatory bed. A 3-6-fold greater dilation of the occluded capillaries in the interalveolar septa was recorded. Some arterioles contained mixed thrombi. A morphometric examination of the control group lung tissue samples revealed that the relative area of thrombi in the samples was 3.538 ± 1.982%, while the average area of thrombi in samples was 1423.5 ± 0.328 µm2 (Table 1). Microscopic investigation of the animals which received SBT-828 revealed predominantly congested capillaries of interalveolar septa as well as of other vessels of the microcirculatory bed. Thickening of the interalveolar septa caused by capillary congestion and swelling was reported. Diapedesis of erythrocytes into interalveolar septa, into the lumen of some alveoli as well as stasis signs and focal hemorrhagic lesions were also recorded. Compared to the control animals, a significant 76.5% reduction in the relative area of thrombi in lung tissue samples and a 79.8% reduction in the average area of thrombi was established (Table 1).
| Compound | Relative area of thrombi, %, (M ± m) | Average area of thrombus per sample, μm2, (M ± m) |
| Control | 3.538 ± 1.982 | 1423.5 ± 0.328 |
| SBT-828 | 0.832 ± 0.132 (р ≤ 0.01) | 287.8 ± 197.2 (р ≤ 0.001) |
| Aspirin | 1.327 ± 0.152 (р ≤ 0.01) | 1337.7 ± 888.5 |
Histological examination of the lung tissue samples obtained from the animals which had received a comparator revealed medium-sized alveoli along with areas of emphysematous and dilated alveoli, alternating with small-sized alveoli which is characterized by markedly congested capillaries of interalveolar septa. White and mixed thrombi adhering to the vascular wall were found in a number of vessels of the microcirculatory bed. A well-pronounced 2-5-fold greater dilation of the occluded capillaries in the interalveolar septa was reported. As compared to the control group, the intake of aspirin led to a statistically significant reduction in the relative area of thrombi in lung tissue samples by 62.5% and to a statistically insignificant reduction by 6% in the average area of thrombi in lung tissue samples (Table 1).
Microtomic sections (4-6 μm wide) were made from paraffin blocks and were then stained with hematoxilin-eosin. Values for relative area of thrombi and average area of thrombus per sample were processed using Statistica/w5.0, basic statistical analysis software for Windows (Stat.Soft. Inc., USA). VideoTestMorpho-4 software was used to perform statistical analysis of histological findings, (n = 10 in all groups).
The electric current which flows through the anode is widely used to induce arterial thrombosis in experiments on rats, as blood platelets play a key role in the development of the lesion [18]. As reported in literature, carotid artery occlusion developed on average within 14.6 min. after passing the electric current through the artery and administering the solvent to the control animals [19]. The oral intake of the test compound two hours before an antiaggregant starts to act, contributed variably to the prevention of total carotid artery occlusion in rats.
The time period of thrombus formation upon administering aspirin at a dose of 20 mg/kg was 15.1 min. which was a statistically insignificant increase of 8% within the time period, as compared to the controls. The administration of SBT-828 at a dose of 47 mg/kg which is similar to 20 mg/kg of acetylsalicylic acid, increased the time period of thrombus formation by 86.7% (p < 0.001). The time period of thrombus formation was 27 min. The dose of aspirin was further increased to 60 and 125 mg/kg and the time period of carotid artery occlusion also increased by 20 (p < 0.05) and 95% (p < 0.001), respectively, as compared to the controls. Moreover, the administration of SBT-828 at a dose of 23 and 35 mg/kg increased the time period during which carotid artery occlusion occurred by 16 (p < 0.01) and 48% (p < 0.05), respectively. ED50 for SBT-828 was 34.3 mg/kg, while for aspirin, it was 61.8 mg/kg (Table 2).
| Compound | Dose (mg/kg ) | The time period of thrombus formation (min) | ED50 (mg/kg ) |
| Control | 14.6 ± 0.74 | ||
| Sbt-828 | 47 | 27.3 ± 1.76 (р ≤ 0.001) | 34.3 |
| 23.5 | 17.0 ± 0.40 (р ≤ 0.05) | ||
| 35.5 | 21.0 ± 0.82 (р ≤ 0.05) | ||
| Aspirin | 20 | 15.1 ± 0.42 | 61.84 |
| 60 | 17.5 ± 0.35 (р ≤ 0.05) | ||
| 125 | 28.5 ± 0.64 (р ≤ 0.001) |
Therefore, novel indole derivative SBT-828 demonstrates an antithrombotic activity in models of arterial thrombosis over aspirin.
Platelet activation is an integral component of the pathophysiology that leads to thrombotic and ischemic diseases such as myocardial infarction, cerebral stroke, and peripheral vascular disease. Platelet inhibition is a major strategy to prevent arterial thrombosis [20]. One of the ways for platelet function inhibition is, inhibition of enzymatic cascades. Indole derivatives have been reported to possess antiaggregant properties, and thus are considered as COX and TXA2 inhibitors [21, 22]. Therefore, aspirin was suggested as a comparator. In vitro comparative studies of antiaggregant activity of a novel indole derivative proved its superiority over aspirin by the time. Consequently, the ability of SBT-828 to prevent mortality of animals in a murine systemic cellular thrombosis model as well as to increase the time period of arterial thrombosis development in an electrically-induced arterial thrombosis model proves that the antithrombotic activity of SBT-828 is probably related to its effect on pathogenetic features of platelet aggregation, and thus it prevents further activation of haemostasis.
Statistical analysis of experimental findings was carried out using Mann-Whitney test (n = 15 in all groups).
ConclusionTop
Histological examination of the animal lung tissue samples revealed that the novel indole derivative which had showed a well-pronounced antiaggregant action in vitro, prevented the death of white outbred mice in a murine systemic collagen-adrenalin-induced thrombosis model. It was proved that SBT-828 prolonged the duration of thrombus formation in rat carotid artery in an electrically-induced arterial thrombosis model. ED50 showed superiority over aspirin that was 1.8 times greater. The antithrombotic action of the novel indole derivative is associated with its potent antiaggregant activity.
Conflicts of interest
The authors declare no conflicts of interest.
Author contribution
All the authors contributed to the development of study design, data analysis and data interpretation. K. Suzdalev synthesized SBT-828 test compound; M. Tyan and A. Kucheryavenko contributed to the experimental work by conducting experiments, analyzing the data obtained in the course of the study and by writing the paper. A. Spasov contributed greatly in developing the design of the experiments and conducting the experiments. A. Kucheryavenko contributed to data analysis and revision of the paper.
ReferencesTop
[1]Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013; 93(1):327–358.Article Pubmed
[2]Jurk K, Kehrel BE. Pathophysiology and biochemistry of platelets. Internist. 2010; 51(9):1086, 1088–1092, 1094.Article Pubmed
[3]Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012; 367(22):2100–2109.Article Pubmed
[4]Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, et al. Double–dose versus standard– dose clopidogrel and high– dose versus low– dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT– OASIS 7): A randomised factorial trial. Lancet. 2010; 376(9748):1233–1243.Article Pubmed
[5]Pais P. P2Y12 inhibitors in acute coronary syndromes: Which and when Natl Med J India. 2013; 26(2):84–90.Article Pubmed
[6]Giannitsis E, Katus HA. Antiplatelet therapy-ticagrelor. Hamostaseologie. 2012; 32:177–185.
[7]Silva MV, Dusse LM, Vieira LM, Carvalho Md. Platelet antiaggregants in primary and secondary prevention of atherothrombotic events. Arq Bras Cardiol. 2013; 100(6):78– 84.Article Pubmed
[8]Dretzke J, Riley RD, Lordkipanidzé M, Jowett S, O'Donnell J, et al. The prognostic utility of tests of platelet function for the detection of 'aspirin resistance' in patients with established cardiovascular or cerebrovascular disease: A systematic review and economic evaluation. Health Technol Assess. 2015; 19(37):1–366.Article Pubmed
[9]Kumar A, Kao J. Platelet resistance to antiplatelet drugs. Resent Pat Cardiovasc Drug Discov. 2009; 4(2):98–108.Pubmed
[10]Zakarija A, Kwaan HC, Moake JL, Bandarenko N, Pandey DK, et al. Ticlopidine and clopidogrel associated thrombotic thrombocytopenic purpura (TTP): Review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989–2008). Kidney Int Suppl. 2009; (112):s20–24.Pubmed
[11]Spasov AA, Suzdalev KF, Кucheryavenko AF, Petrov VI, Minkin VI. Compound with antiplatelet activity. RU №2486182 C2. 2013.
[12]DiMinno G, Silver MJ. Mouse antithrombotic assay: A simple method for the evaluation of antithrombotic agents in vivo. Potentiation of antithrombotic activity by ethyl alcohol. J Pharmacol Exp Ther. 1983; 225(1):57–60.Article Pubmed
[13]Sarcisov DS, Perov YL. Microscopic technics 7– 47, Medicine, Moscow. 1996.
[14]Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991; 75(1):8–14.Article Pubmed
[15]Guarneri L. A new model of pulmonary microembolism in the mouse. J Pharmacol Methods. 1988; 20(2):161–167.Article Pubmed
[16]Furie B, Furie BC. Thrombus formation in a living mouse. Pathophysiol Haemost Thromb. 2006; 35(1–2):1–4.Article Pubmed
[17]Murina MA, Fesenko OD, Sergienko VI, et al. Antithrombotic activity N,N– dichlortaurine in vivo in a model thrombosis in mice. Bull Exper Biol And Med. 2002; 134(7):44–47.
[18]Niitsu Y, Sugidachi A, Ogawa T. Repeat oral dosing of prasugrel, a novel P2Y12 receptor inhibitor, results in cumulative and potent antiplatelet and antithrombotic activity in several animal species. Europ J of Pharmacol. 2007; 579(1-3):276–282.Article Pubmed
[19]Schumacher WA, Heran CH, Steinbacher TE, Megill JR, Bird JE , et al. Thrombin inhibition compared with other antithrombotic drugs in rats. Thromb Res. 1992; 68(2):157–166.Article Pubmed
[20]Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007; 357(24):2482–2494.Article Pubmed
[21]Bhana N, McClellan KJ. Indobufen: An updated review of its use in the management of atherothrombosis. Drugs Aging. 2001; 18(5):369–388.Article Pubmed
[22]Barillà F, Pulcinelli FM, Mangieri E, Torromeo C, Tanzilli G, et al. Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets. 2013; 24(3):183–188.Article Pubmed



