Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 1, Issue 10, December 2013, Pages 226-229
Case reportOpen Access
Hyperpigmentation of the tongue, palms and soles: Rare side effect of capecitabine
- 1 Department of Oncology, King Faisal Specialist Hospital & Research Centre, Jeddah, Saudi Arabia
- 2 College of Medicine, Al-Faisal University, Saudi Arabia
- 3 Department of Medical Oncology, National Cancer Institute, Cairo University, Egypt
*Corresponding author: Jamal Zekri, Department of Oncology, King Faisal Specialist Hospital and Research Centre, Jeddah, Saudi Arabia, Tel.: +966 266 77777 (ext. 65066); Fax: +966 266 77777 (Ext 64030). E-mail: jmzekri@hotmail.com
Received 12 August 2013 Revised 3 November2013 Accepted 10 November 2013 Published 17 November 2013
DOI: http://dx.doi.org/10.14312/2052-4994.2013-34
Copyright: ©2013 Zekri J, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Cutaneous side effects of capecitabine include hand foot syndrome which manifests as erythema, swelling and/or desquamation. We report a case on capecitabine treatment presenting with unusual hyperpigmentation of the tongue and skin of the palms and soles. This is a rare manifestation of capecitabine toxicity in darker skin patients. Clinicians and health care workers in oncology should be aware of this potential side effect.
Keywords: capecitabine; tongue; palms; soles; hyperpigmentation
IntroductionTop
Capecitabine is an oral antineoplastic agent that undergoes 3 steps enzymatic conversion to 5-fluorouracil (5-FU) [1]. It is approved for the treatment of colorectal and metastatic breast cancers. Dose limiting side effects of capecitabine include mucositis, hyperbilirubinemia, diarrhea, myelosuppression and hand-foot syndrome (HFS). All grades HFS manifest in 49% and grade III/IV in 11% of patients receiving capecitabine for advanced breast cancer [2]. Hyperpigmentation of palms and/or sole is not classic component of HFS and pigmentation of the tongue is extremely rarely associated with capecitabine use.
The FDA-approved dose (2500 mg/m2/day) can lead to unacceptable toxicity in many patients. Dose interruptions and reductions are necessary in approximately one-third of patients, and drug discontinuation has been required in as many as 17% of patients in clinical trials (range 7%–17%) [3]. Dose reduction and lower starting dose with tolerance guided subsequent escalation improves tolerability without compromising efficacy [4, 5].
We present a case presenting with hyperpigmentation of the tongue and skin of the palms and soles while on capecitabine treatment.
Case reportTop
59 year old woman with metastatic breast carcinoma to the bones started first cycle of capecitabine at a reduced dose of 1500 mg twice daily (BID) for 14 days every 21 days. Starting capecitabine at lower dose followed by escalation to standard dose according to tolerability is a standard practice at our institution. She tolerated first cycle well. The second cycle was prescribed at full recommended dose of 2000 mg BID (equivalent to 1250 mg/m2 BID). On presentation for the third cycle she was found to have generalized hyperpigmentation of the skin of palms that was more apparent on the palmar surface of the fingers (Figure 1) and hyperpigmentation of the skin of soles that was more apparent along the creases and pressure areas of the sole and toes with relative sparing of non-pressure areas on the soles (Figure 2). Other manifestations of HFS were not present, namely erythema, swelling, numbness, tingling, discomfort, pain, blistering, desquamation and ulceration.
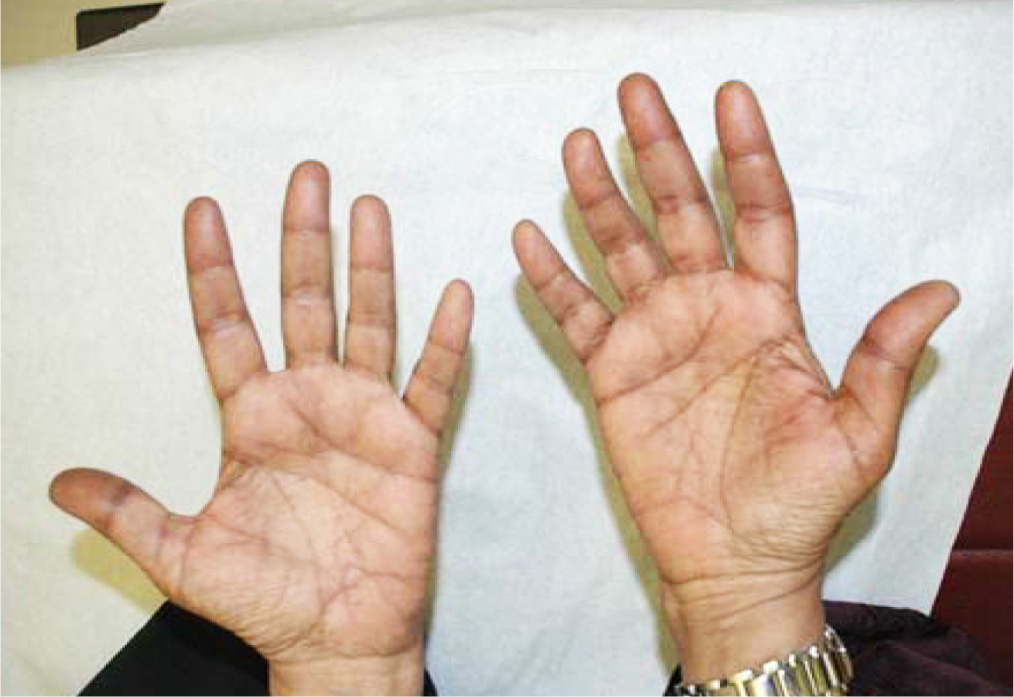
Figure 1 Hyperpigmentation of the skin of palms.
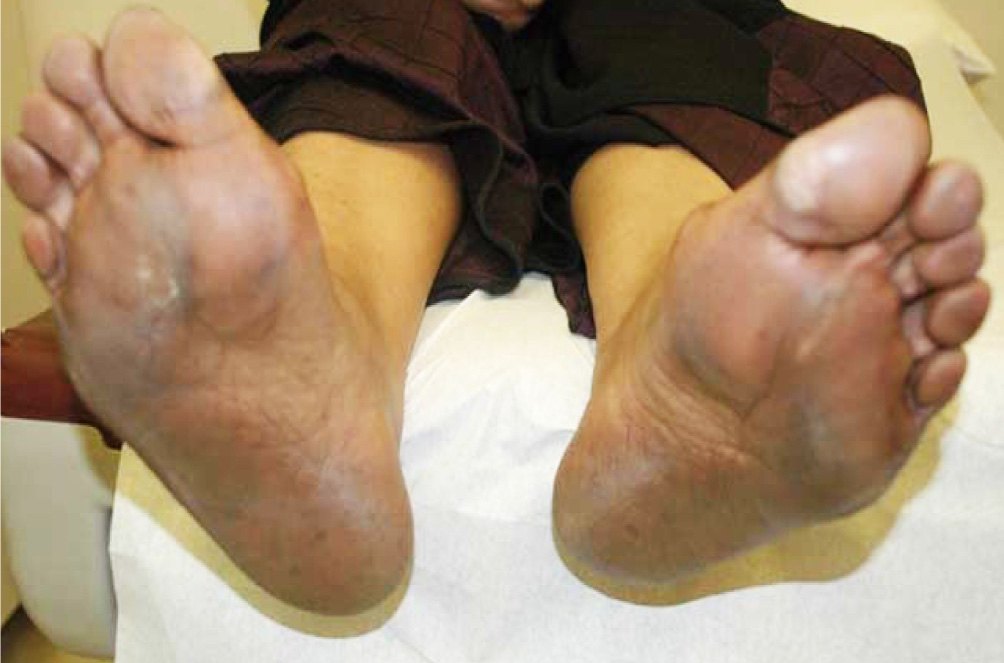
Figure 2 Hyperpigmentation of the skin of soles that is more apparent along the creases and pressure areas of the sole.
The tongue showed painless well demarcated hyper-pigmented non-raised macules (Figure 3). The rest of the tongue and oral cavity was normal and in particular there was no mucositis. The patient was reassured and advised to use emollients. The third cycle of capecitabine was prescribed at reduced dose of 1500 mg BID. Examination 3 weeks later showed similar findings. At the same time a decision was made to stop capecitabine due to deterioration of metastatic disease. Follow up 10 weeks later showed complete resolution of all palms, soles and tongue lesions.
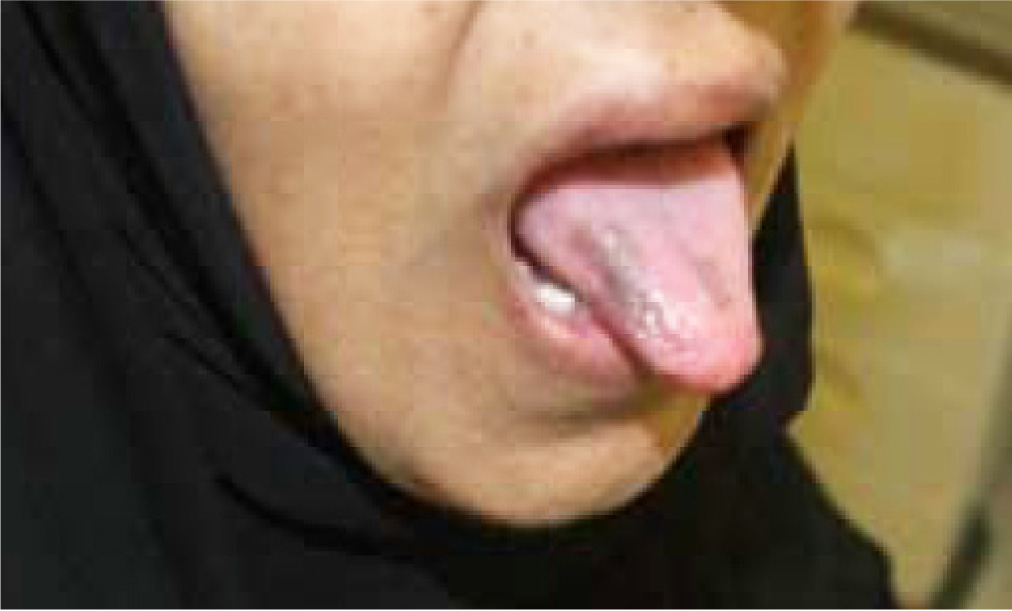
Figure 3 Painless well demarcated hyper-pigmented non-raised macules in the tongue.
Results and discussionTop
Cutaneous hyperpigmentation has been observed and reported in patients receiving cytotoxic chemotherapy. It may affect any part of the tegument including hair, nails and mucous membranes and can be either diffuse or localized and may have a distinctive pattern [6] (Table 1).
| Pattern of hyperpigmentation | Chemotherapeutic agent |
| Acral | Tegafur and Capecitabine |
| Diffuse | Busulphan, Cyclophosphamide, Hydroxyurea and Methotrexate |
| Patchy | 5FU |
| Lentigo and eruptive naevi | Oral and intravenous fluoropyrimidines |
| Flagellate | Bleomycin |
| Transverse bands | Cyclophosphamide |
| Supravenousserprntine | Paclitaxel, Docetaxel, Fotemustine, Vinorelbine and Vincristine |
HFS is the most recognized cutaneous side effect of capecitabine. The clinical manifestations of capecitabine induced HFS classically start with bilateral numbness and tingling of the palms of the hands and soles of the feet followed by erythema. The palms and soles may become painful with variable degrees of swelling particularly on the pads of the fingers. If the condition progresses, blisters, desquamation and fissures develop. Cutaneous hyperpigmentation is also associated with use of capecitabine. The underlying pathogenesis of hyperpigmentation remains unknown in most cases but is likely to be to different depending on the drug. Proposed mechanisms include: (a) Direct toxic effect on melanocytes with subsequent melanin synthesis stimulation, (b) Hypersecretion of adrenocorticotropic hormone and melanocyte-stimulating hormone as a result of adrenal toxicity, (c) Deficiency in tyrosinase inhibitors, (d) Formation of stable drug-melanin complexes and (e) Post-inflammatory pigmentation following toxicity affecting keratinocytes with or without photosensitivity [7].
Capecitabine associated cutaneous hyperpigmentation can be general or more frequently, localized in palms and soles. It is more obvious in dark-skinned patients, Asians, or blacks. Less frequently, pigmentation may be found in photo-exposed areas, on venous courses. Eruptive lentiginosis on the palms and soles and re-pigmentation of vitiligohavealso been reported [8, 9, 10]. Nail changes reported with the use of capecitabine include: periungual pyogenic granuloma like lesions, Beau’s lines, onycholysis, onychomadesis and melanonychia [11, 12].
Based on our experience in the Middle East, HFS usually presents with classic manifestations as described above. However, only in few cases, we noticed the occurrence of hyperpigmentation of the palms and soles. Our patient is from Arabic Middle Eastern ethnic background with brown skin (skin type IV-V on Fitzpatrick scale). She developed hyperpigmentation of hands and feet without other classic manifestations of HFS. This raises the question whether these changes are a form of early grade of HFS or that these changes are isolated unrelated events resulting in transient pigmentation.
There are few case reports in the literature describing similar findings in black skin African-American patients receiving infusional 5-FU and capecitabine [13, 14]. In most of these reports hyperpigmentation was associated with development of HFS. For example one described palmar and plantar hyperpigmentation in a Ghanese woman receiving capecitabine. Hyperpigmentation preceded signs and symptoms of HFS [15].
A report from India presented a 49 year old lady with capecitabine induced hyperpigmentation and dryness of skin with fissuring of palms and soles associated with loss of nails and ulcerations over the great toes. The report did not indicate the ethnicity of the patient. However, the accompanied clinical photographs indicate a patient with skin type IV-V [16].
Similarly, Vickers et al reported on 3 patients (Indian, Asian and Aboriginal) who developed moderate to severe HFS requiring delay and dose reduction. In every case, toxic side effects were preceded by hyperpigmentation of hands and feet [17].
A case report from Spain described diffuse hyperpigmentation of the palms and soles more noticeable in the skin folds in a 46 year old lady with skin type III receiving capecitabine for treatment of breast cancer. Hyperpigmentation was associated with dysesthesia which is an early sign of HFS. No hyperpigmentation was observed in the mucosa or elsewhere [18].
Our patient also developed similar hyperpigmentation but which was more apparent with the creases and pressure areas of the sole and toes with obvious relative sparing of non-pressure areas on the soles. Hyperpigmentation of palms was also more apparent on the pads of the fingers probably reflecting more usage friction at these sites. The appearance of pigmentation at sites of friction and trauma is well recognized. It has been speculated that this could result from increased local blood flow and subsequent drug deposition [17].
The absence of classic signs and symptoms of HFS in our case suggests that hyperpigmentation can present as an isolated event not related to HFS. Another explanation is that hyperpigmentation is an early sign and in our case did not progress to manifest other classic features. In addition, our patient developed hyper-pigmented macules on the tongue.
Capecitabine induced tongue pigmentation may not be uncommon but is probably underreported. Moreover, systematic evaluation of oral cavity in these patients is not a routine practice. After extensive literature search, we found only 3 papers reporting capecitabine induced pigmentation of the tongue. One reported on a 59 year old man with adenocarcinoma of stomach receiving capecitabine as adjuvant chemotherapy. After two cycles of therapy, patient developed hyperpigmentation on hands and feet and hyper-pigmented spots on the dorsum of tongue. The accompanied clinical photographs indicated an Indian origin patient with skin type V [19].
The second report was from Spain in 58 year old woman undergoing chemotherapy with capecitabine. She developed multiple pigmented macules on the skin and tongue, and generalized hyperpigmentation, which finally faded after the drug discontinued [20]. The third report was of a 36 year old African-American woman on capecitabine for metastatic breast cancer who developed large geographic area of hyperpigmentation of the tongue and gums associated with hyper-pigmented macules on the palms, extremities and trunk [21]. Fluoropyrimidine induced hyperpigmentation of palms and soles have sparsely been reported in patients with fairer skin. It has been reported in a patient from Korea on capecitabine treatment in association with pain and desquamation [22].
Hyperpimentation of palms and solesin darker skin population may be an early sign of HFS or may appear as an isolated finding without further progression.
ConclusionTop
Hyperpigmentation of the tongue, hands and feet is a rare but recognized side effect of capecitabine chemotherapy in darker skin patients. Clinicians and health care workers in oncology should be aware of this potential finding which may or may not progress to HFS.
Conflict of interest
The authors wish to express that they have no conflict of interest.
ReferencesTop
[1] Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, et al. (1998) Design of a novel oral fluoropyrimidinecarbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1278. Article Pubmed
[2] Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. Article Pubmed
[3] Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, et al. (2001) Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 12:1247–1254. Article Pubmed
[4] Hennessy BT, Gauthier AM, Michaud LB, Hortobagyi G, Valero V (2005) Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer:retrospective analysis of patients treated at M. D. Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol 16:1289–1296. Article Pubmed
[5] Leonard R, Hennessy BT, Blum JL, O'Shaughnessy J (2011) Dose-adjusting capecitabine minimizes adverse effects while maintaining efficacy: a retrospective review of capecitabine for metastatic breast cancer. Clin Breast Cancer 11:349–356. Article Pubmed
[6] Balagula E, Lacouture ME (2010) Dermatologic toxicities.The MASCC textbook of cancer supportive care and survivorship. Springer, pp. 361–380. Article
[7] Dereure O (2001) Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol 2:253–262. Article Pubmed
[8] Bogenrieder T, Weitzel C, Schölmerich J, Landthaler M, Stolz W (2002) Eruptive multiple lentigo-maligna-like lesions in a patient undergoing chemotherapy with an oral 5-fluorouracil prodrug for metastasizing colorectal carcinoma: a lesson for the pathogenesis of malignant melanoma? Dermatology 205:174–175. Article Pubmed
[9] Fukushima S, Hatta N (2004) Atypical moles in a patient undergoing chemotherapy with oral 5-fluorouracil prodrug. Br J Dermatol 151:698–700. Article Pubmed
[10] Schmid-Wendtner MH, Wendtner CM, Volkenandt M, Heinemann V (2001) Clinical picture: leopard-like vitiligo with capecitabine. Thelancet 358:1575.. Article Pubmed
[11] Gilbar P, Hain A, Peereboom VM (2009) Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract 15:143–155. Article Pubmed
[12] Paravar T, Hymes SR (2009) Longitudinalmelanonychia induced by capecitabine. Dermatol Online J 15:11. Article Pubmed
[13] Saif MW, Elfiky AA (2007) Identifying and treating fluoropyrimidine-associated hand-and-foot syndrome in white and non-white patients. J Support Oncol 5:337–343. Article Pubmed
[14] Narasimhan P, Narasimhan S, Hitti IF, Rachita M (2004) Serious hand-and-foot syndrome in black patients treated with capecitabine: report of 3 cases and review of the literature. Cutis 73:101–106. Article Pubmed
[15] vvanTienhoven G, Wilmink JW (2011) A woman with palmar and plantar hyperpigmentation. Ned Tijdschr Geneeskd 155:A4100. Article Pubmed
[16] Surjushe A, Vasani R, Medhekar S, Thakre M, Saple DG (2009) Hand-foot syndrome due to capecitabine. Indian J Dermatol 54:301–302. Article Pubmed
[17] Vickers MM, Easaw JC (2008) Palmar-plantar hyperpigmentation with capecitabine in adjuvant colon cancer. J Gastrointest Cancer 39:141–143.. Article Pubmed
[18] Vázquez-Bayo C, Rodríguez-Bujaldón AL, Jiménez-Puya R, Galán-Gutiérrez M, Moreno-Giménez JC (2007) Capecitabine-induced hyperpigmentation. Actas Dermosifiliogr 98:491–493. Article Pubmed
[19] Vasudevan B (2010) An unusual case of capecitabine hyperpigmentation: Is hyperpigmentation a part of hand-foot syndrome or a separate entity? Indian J Pharmacol 42:326–328. Article Pubmed
[20] Villalón G, Martín JM, Pinazo MI, Calduch L, Alonso V, et al. (2009) Focal acral hyperpigmentation in a patient undergoing chemotherapy with capecitabine. Am J Clin Dermatol 10:261–263. Article Pubmed
[21] Pui JC, Meehan S, Moskovits T (2002) Capecitabine induced cutaneous hyperpigmentation: report of a case. J Drugs Dermatol 1:202–205. Article Pubmed
[22] Lee SD, Kim HJ, Hwang SJ, Kim YJ, Nam SH, et al (2007) Hand-foot syndrome with scleroderma-like change induced by the oral capecitabine: a case report. Korean J Intern Med 22:109–112. Article Pubmed



