Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 3, Issue 2, February 2015, Pages 15–19
Original researchOpen Access
The positive sentinel lymph node biopsy- can we predict which patients will benefit from further surgery?
-
Isabella Dash1,*
 ,
Donna Egbeare1,
Megan Straiton1,
Katrina Schwodler1,
Gordon Taylor2,
Dorothy A Goddard1,
Jamie McIntosh1 and
Richard J Sutton1
,
Donna Egbeare1,
Megan Straiton1,
Katrina Schwodler1,
Gordon Taylor2,
Dorothy A Goddard1,
Jamie McIntosh1 and
Richard J Sutton1
- 1 Royal United Hospital Bath NHS, Foundation Trust, Combe Park, Bath, Somerset BA1 3NG, United Kingdom
- 2 University of Bath, Claverton Down, Bath, BA2 7AY, United Kingdom
*Corresponding author: Isabella Dash, General Surgery, Royal United Hospital Bath NHS, Foundation Trust, Combe Park, Bath, Somerset BA1 3NG, United Kingdom. Tel.: +44 7977 127711; Email: izzydash@hotmail.com
Received 20 November 2014 Revised 9 January 2015 Accepted 17 January 2015 Published 25 January 2015
DOI: http://dx.doi.org/10.14312/2052-4994.2015-3
Copyright: © 2015 Dash I, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Introduction: Although sentinel lymph node biopsy (SLNB) is the gold standard for clinical and radiological negative axillae in breast cancer, the subsequent management of positive nodes is currently under scrutiny with the Z0011 trial publication causing much debate. Our aim was to determine whether there were any predictive factors in our cohort of patients that could assist in the decision of whether the patient required a further axillary lymph node clearance. Methods: A retrospective analysis of a prospectively maintained database of patients who underwent SLNB and their histology was performed. Univariate and multivariate analysis was performed to identify any predictive factors. Results: Our Breast Unit performed 457 SLNB over the three years. The mean age of patients was 61.9 years (range 31-89 years). Of these 457 SLNB, 122 (26.7%) were positive for metastatic involvement, and of these patients only 34% were found to have further lymph node involvement after axillary lymph node clearance (ALNC). Only 8% of our total patient cohort had non sentinel node involvement at completion ALNC. Using univariate analysis, lymphovascular invasion (p=0.009), grade (p=0.007) and size (p=0.006) were all significant predictors for having a positive ALNC. Conclusion: Only 8% of the total number of patients had an additional positive lymph node found in their completion axillary clearance. Our results add to this existing knowledge on the subject, and show that there are certain factors which should be carefully considered pre-operatively in order to help inform patient and surgeon choice regarding management of the axilla.
Keywords: sentinel lymph node biopsy; axillary lymph node clearance; breast neoplasms; pre-operative staging; ultra-sound guided biopsy
IntroductionTop
Sentinel lymph node biopsy (SLNB) has become the accepted standard for staging the axilla in the treatment of patients with clinical and radiological lymph node negative breast cancer. However the management of those patients with a positive sentinel node still remains axillary lymph node clearance (ALNC). According to The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial results [1], completion ALNC could be avoided safely in many of these sentinel lymph node positive breast cancer patients, therefore avoiding the increased risks of lymphedema and other morbidities associated with ALNC. The ACOSOG Z0011 trial suggested that there was no inferiority of sentinel lymph node dissection alone compared with completion ALNC in terms of local recurrence rates and overall disease mortality in women with T1 or T2 tumours who undergo breast conservation surgery followed by whole breast radiotherapy.
However, there have been criticisms of the trial [2], especially with regards to the inclusion criteria and as to whether the lower axilla was included in the radiotherapy field for the breast irradiation after wide local excision. In addition it may be difficult to accurately extrapolate the results of this study to many of the patients treated in a UK Breast Unit, as many of these patients would not fall within the inclusion criteria for Z0011, especially those who have a mastectomy.
Patients in our unit who have clinically negative (no palpable axillary lymph nodes) or radiologically negative (ultrasound determined normal morphology or histologically normal following ultrasound guided core biopsy) undergo SLNB as per current United Kingdom guidelines; Association of Breast Surgeons [3] and National Institute for Clinical Excellence [4].
We wished to identify any factors that could be used to predict which patients with a positive SLNB would benefit from further ALNC, and which patients could avoid undergoing the procedure unnecessarily.
MethodsTop
Using a prospectively maintained database of all the patients who had undergone SLNB between 1st January 2009 and 31st December 2011, we retrospectively analysed the histology (Haematoxylin and Eosin staining) results from Anglia Ice, our Trust's computerised results software. Patients who underwent Neo-adjuvant chemotherapy were excluded from this cohort of patients as during this time period, our unit was reviewing the management pathway of these patients. We compared SLNB results with subsequent results from the patients who underwent an ALNC.
We used SPSS Version 20 for univariate analysis, with chi-squared analysis or Fisher’s exact test depending on sample size, and logistic regression to identify whether any multiple factors were significant.
ResultsTop
Our Breast Unit performed 457 SLNB over the three years. The mean age of patients was 61.9 years (range 31-89 years). Of these 457 SLNB, 122 (26.7%) were positive for metastatic involvement. As expected, the original tumour histology reflected that of the general population with 76% having invasive ductal carcinoma, the remaining histological breakdown is shown in Table 1. Table 2 shows further patient and tumour characteristics.
| Type of tumour | Population |
| Invasive ductal carcinoma | 93 (76%) |
| Invasive lobular carcinoma | 17 (14%) |
| Mucinous | 2 (1.7%) |
| Tubular | 3 (2.5%) |
| DCIS | 2 (1.7%) |
| LCIS | 1 (0.8%) |
| Mixed | 4 (3.3%) |
Note: Total number of patients and percentages divided by each type of tumour.
| Characteristics | Number of Patients |
| Size | |
| T1 (<2cm) | 55 |
| T2 (>2 -<5cm) | 52 |
| T3 (>5cm) | 15 |
| Grade | |
| 1 | 19 |
| 2 | 84 |
| 3 | 19 |
| LV Invasion | |
| Yes | 77 |
| No | 45 |
| ER positive | |
| Yes | 96 |
| No | 26 |
| Her 2 Positive | |
| Yes | 73 |
| No | 49 |
Of the patients with a positive SLNB, 68% of patients (n=83) had macrometastases (≥2mm), 29.5% (n=36) had micrometastases (0.2-2mm) and 2.5% (n=3) had isolated tumour cells (ITC) (≤0.2mm) [5]. The majority of patients had Grade 2 tumours, 77 patients had Lymphovascular invasion (LVI) (63%). In contrast; of the patients with a negative SLNB, 96 (30%) had LVI in their tumour histology.
Ninety percent (110/122) of patients with a positive SLNB subsequently underwent an ALNC (Figure 1), 34% of these had a positive LN found at histological examination of the axillary clearance (n=37). Of the 12 patients who did not undergo further axillary surgery, for 5 it was an MDT decision, 4 patients decided that they did not want further axillary surgery, and 1 was treated with radiotherapy to the axilla. For one patient the positive SLN was extra axillary, located in the tail of the breast, with the axillary nodes appearing normal on detailed ultrasound scanning of the axilla. In the last case the patient had an ALNC previously. Figure 2 shows the original size of SLNB metastases in the patients who underwent a further ALNC, with the majority of patients (92%; n=34) with a subsequent positive ALNC, having macrometastases on their original SLNB.
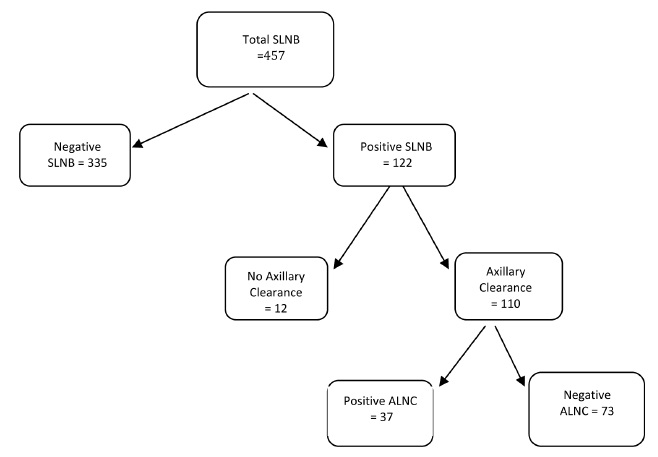
Figure 1 Flow diagram showing numbers of patients and subsequent histology.
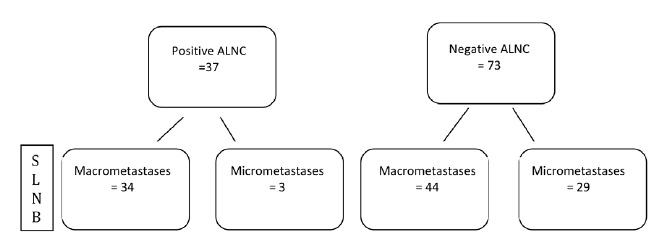
Figure 2 Flow diagram showing size of SLNB metastases divided by ALNC findings.
Our yield from ALNC had a mean number of 17 lymph nodes retrieved in positive ALNC (range 1-36) and 13 in negative ALNC (range 2-26). We also reviewed the patients whose SLNB histology showed extra-nodal spread, one of the exclusion criteria for Z0011. This was identified in 28 patients out of the 83 with macrometastases (33%). Eighteen (64%) of these had positive ALNC. Two patients did not undergo an ALNC.
We further analysed the data to try and identify which patients with a positive SLNB were most likely to have further axillary lymph node disease. Using univariate analysis (Table 3), lymphovascular invasion (p=0.009), Grade 2 (p=0.007) and size of between 2 and 5cm (p=0.006) were all significant predictors for having a positive ALNC, however age (p=0.86), oestrogen receptor status (p=0.38) and human epidermal growth factor receptor 2 (Her2) status were not significant (p=0.84).
Logistic regression (Table 4) identified size of tumour (T2; 2-5cm) (p=0.046) and Grade 2 (p=0.047) as significant in identifying those requiring ALNC. No other factors were significant.
| Variable | Chi2 value |
| Size (T2) | P = 0.006 |
| Grade (2) | P = 0.007 |
| Lymphovascular Invasion | P=0.009 (Fisher’s exact test) |
| Oestrogen receptor positivity | P=0.376 (Fisher’s exact test) |
| HER-2 positivity | P=0.849 |
| Variable | Logistic Regression |
| Size (T2 2-5cm) | P=0.046 |
| Grade (2) | P=0.047 |
| Lymphovascular Invasion | P=0.53 |
| Oestrogen Receptor Positivity | P=0.39 |
| HER2 Positivity | P=0.62 |
DiscussionTop
There is no doubt that SLNB should be the primary axillary surgery in breast cancer patients with clinical and radiological node negative axillae, the NSABP B32 trial [6] has confirmed that there is no difference in overall survival and disease free survival between sentinel node negative patients undergoing SLNB compared to those that underwent completion ALNC in their 8 year follow up.
The other question that needs answering is; what should be done with the patient who has micrometastases in their SLNB? There are trials which have tried to address this, the IBCSG 23-01 trial showed no significant difference in recurrence or 5 year disease free survival when patients with one or more SLN with a micrometastasis were randomized to ALNC or not [7]. Similarly, the AATRM study did not show any differences in disease free survival or cancer related deaths when patients with sentinel node micrometastases were randomized to ALNC or clinical follow up [8].
The St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013 also acknowledged that the Z0011 trial has shown that patients undergoing breast conservation therapy and whole breast radiation do not require ALNC for up to two SLN with macrometastases [9].
As such, the American Society of Clinical Oncology updated its guidelines in March 2014 [10] to recommend that if women are undergoing breast conservation therapy with whole breast irradiation, ALNC was not required for women with up to two positive SLNB.
Systematic reviews of current randomised controlled trials and observational studies have shown no inferiority in overall survival and disease free survival [11] in patients undergoing SLNB alone for node positive SLNB, and no inferiority in axillary recurrence rate [12]. The reduction in morbidity for those patients solely undergoing SLNB is significant. The limitations of performing prospective studies and trials to address the node positive SLNB and further management is well documented, with low accrual rates and reduced disease events and deaths affecting sample sizes.
Until there are more prospective randomised trials, retrospective cohort studies such as this study will help fill the gap in providing further strength and assisting multi-disciplinary team (MDT) decisions as to how best manage their patients with a positive SLNB, especially in the case of the patient with a micrometastasis or ITC. A large French [13] multicentre retrospective cohort study with over 8000 patients concluded that there was no difference in overall and disease free survival in patients who had micrometastases and ITC compared to those who had node negative SLNB, however there was an increased frequency of axillary recurrence rate.
Similarly to our findings, although on a slightly smaller scale a Turkish group [14] found that tumour size and lymphovascular invasion were significant primary tumour related prognostic determinants.
Alternatively, there are six nomograms that exist to aid prediction of lymph node involvement – Cambridge, Memorial Sloan-Kettering Cancer Center (MSKCC), Mayo, MDA, Tenon and Stanford [15, 16]. They have been used in different populations and it is still unclear which is the best and would be most suitable to a UK population. The Stanford calculator [17] only uses three variables, lymphovascular invasion, grade and size of tumor, similar to our findings in this cohort of patients. Their accuracy rate was found to be 77%. Unfortunately, both the MSKCC and the Stanford calculators (the only online calculators) have limitations in that their usefulness is limited to patients with micrometastases and isolated tumor cells [18]. Currently these nomograms are not used routinely in many breast units.
Our results add to the existing knowledge on this subject, and show that there are certain factors which should be carefully considered pre-operatively in order to help inform patient and surgeon choice regarding management of the axilla. However it is important to note that these results are dependent on accurate pre-operative axillary staging with ultrasound and ideally hollow needle core biopsy pre-operatively, although even fine needle aspiration (FNA) has been shown to be highly specific and sensitive [19].
ConclusionTop
In our series 27% of the SLNBs undertaken were positive for metastatic spread, and of these patients only 34% were found to have further lymph node involvement after ALNC. When considering the whole cohort of patients only 8% had non sentinel node involvement at completion ALNC. Our results show that the majority of our patients having a completion ALNC do not benefit from this additional surgery; because in the majority of cases, the remaining lymph nodes were all free of malignancy. Lymphovascular invasion seems to be an independent predictive factor for positive ALNC, with size of tumour and grade also being significant. However, despite a large total population, the overall group sizes are small, leading to poor statistical results. This is not enough to base a pre-operative decision on, but may allow for selectively choosing cases that may benefit from intra-operative analysis (frozen section/one step nucleic –acid amplification (OSNA)) for those cases which have the predictive factors suspicious for requiring an ALNC, as well as allowing improved patient pre-operative information and increased theatre efficiency. This information can be used, together with emerging guidelines, to assist the MDT and patient, into making the appropriate decision.
Conflict of interest
None of the authors declare any conflict of interest.
ReferencesTop
[1] Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, et al. (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6): 569–575. Article Pubmed
[2] Morrow M, Giuliano AE (2011) To cut is to cure: can we really apply Z11 in practice Ann Surg Oncol 18(9):2413–2415. Article Pubmed
[3] Association of Breast Surgery at Baso 2009 (2009) Surgical guidelines for the management of breast cancer. Eur J Surg Oncol 35 Suppl 1:1–22. Article Pubmed
[4] Harnett A, Smallwood J, Titshall V, Champion A (2009) Diagnosis and treatment of early breast cancer, including locally advanced disease—summary of NICE guidance. BMJ 338:b438. Article Pubmed
[5] American Joint Committee on Cancer, Breast Cancer Staging, Financial support for AJCC 7th Edition Staging Posters provided by the American Cancer Society. Accessed December 2014: Article
[6] Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, et al. (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(10):927–933. Article Pubmed
[7] Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, et al. (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol 14(4):297–305. Article Pubmed
[8] Solá M, Alberro JA, Fraile M, Santesteban P, Ramos M, et al. (2013) Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol 20(1):120–127. Article Pubmed
[9] Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. Article Pubmed
[10] Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, et al. (2014) Sentinel lymph node biopsy for patients with early stage breast cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 32:1365–1383. Article
[11] Ram R, Singh J, McCaig E (2014) Sentinel node biopsy alone versus completion axillary node dissection in node positive breast cancer: systematic review and meta-analysis. Inter J Breast Cancer 513780. Article
[12] Francissen CM, Dings PJ, van Dalen T, Strobbe LJ, van Laarhoven HW, et al. (2012) Axillary recurrence after a tumor-positive sentinel lymph node biopsy without axillary treatment: a review of the literature. Ann Surg Oncol 19(13):4140–4149. Article Pubmed
[13] Houvenaeghel G, Classe JM, Garbay JR, Giard S, Cohen M, et al. (2014) Prognostic value of isolated tumor cells and micrometastases of lymph nodes in early-stage breast cancer: a French sentinel node multicenter cohort study. Breast 23(5):561–566. Article Pubmed
[14] Postaci H, Zengel B, Yararbas U, Uslu A, Eliyatkin N, et al. (2013) Sentinel lymph node biopsy in breast cancer: predictors of axillary and non-sentinel lymph node involvement. Balkan Med J 30(4):415–421. Article
[15] Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, et al. (2003) A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 10(10):1140–1151. Article Pubmed
[16] Zhu L, Jin L, Li S, Chen K, Jia W, et al. (2013) Which nomogram is best for predicting non-sentinel lymph node metastasis in breast cancer patients? A meta-analysis. Breast Cancer Res Treat 137(3):783–795. Article Pubmed
[17] Kohrt HE, Olshen RA, Bermas HR, Goodson WH, Wood DJ, et al. (2008) New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer 8:66. Article Pubmed
[18] Tanaka S, Sato N, Fujioka H, Takahashi Y, Kimura K, et al. (2013) Validation of online calculators to predict the non-sentinel lymph node status in sentinel lymph node-positive breast cancer patients. Surg Today 43(2):163–170. Article Pubmed
[19] Gilani SM, Fathallah L, Al-Khafaji BM (2014) Preoperative fine needle aspiration of axillary lymph nodes in breast cancer: clinical utility, diagnostic accuracy and potential pitfalls. Acta Cytol 58(3):248–254. Article Pubmed


