Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 6, Issue 3, May 2018, Pages 18–24
Original researchOpen Access
Clinical study of the treatment of multiple brain metastases from adenocarcinoma of the lung by simultaneous integrated boost intensity-modulated radiotherapy
- 1 Department of Radiation Oncology, Shandong cancer hospital affiliated to Shandong University, Shandong Academy of Medical Sciences, Jinan, Shandong, China 250117
*Corresponding author: Jianbin LI, Department of Radiation Oncology (Chest Section), Shandong cancer hospital affiliated to Shandong University, Shandong Academy of Medical Sciences, Road Jiyan 440, Jinan 250117, China. Phone: +86-531-67626130; Fax: +86-531-67626130; E-mail: lijianbin@msn.com
Received 5 March 2018 Revised 17 April 2018 Accepted 25 April 2018 Published 2 May 2018
DOI: http://dx.doi.org/10.14312/2052-4994.2018-3
Copyright: © 2018 Shao Q, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Objective: To investigate the effects of simultaneous integrated boost intensity-modulated radiotherapy (SIB) and sequential boost conformal radiotherapy (CRT) in treatment of multiple brain metastases from lung adenocarcinoma. Methods: Forty-two patients with multiple brain metastases from lung adenocarcinoma were analyzed. The epidermal growth factor (EGFR) mutations were negative, ECOG (Eastern Cooperative Oncology Group) were not more than two, the diameters of brain metastases were from 0.5cm to 3.0cm and the numbers of brain metastases were from three to five. The target volume of radiotherapy included whole brain and brain metastatic tumor. The radiation models were divided into SIB in 26 cases and CRT in 16 cases. The median radiation dose of whole brains in SIB and CRT groups were 40Gy, and the brain metastases tumor were 55Gy, 60Gy respectively, and the median number of radiotherapy for 22 times, 30 times. Thirty one patients followed by pemetrexed maintenance chemotherapy (PMC) or docetaxel maintenance chemotherapy (DMC) and 11 patients received no maintenance chemotherapy (NMC). Results: The 1-, 3-, and 5-year survival rates of whole group were 92.8%, 23.8%, and 4.8%, respectively. The median survival time of SIB and CRT groups were 36 months, 29 months, respectively. The SIB group was better than CRT group, and PMC was better than DMC or NMC. Multiple factors analyses showed ECOG, doseand times of brain metastatic tumor radiotherapy, and cycles of PMC were independent risk factors for survival time. The late main adverse reaction was hypomnesia. Conclusion: SIB was superior to CRT, and PMC could improve survival time.
Keywords: lung cancer; adenocarcinoma; brain metastases; conformal radiotherapy; maintenance chemotherapy; concurrent chemoradiotherapy
IntroductionTop
The brain is one of the most common metastatic organs for lung cancer [1]. Brain metastases (BM) account for 10% of lung cancer (NSCLC) patients at initial presentation [2], with another 40–60% developing BM during the course of their disease [3]. For patients with brain metastases from lung cancer, the median survival time is one to two months if they are not treated or best support treated [4]. The survival time of patients can be prolonged through treatment [4-6]. Radiotherapy is the major and effective treatment for multiple brain metastases, and the whole brain radiotherapy (WBRT) is the standard treatment for multiple brain metastases, while radiotherapy has side-effect on patients’ life quality. It is common that lung adenocarcinoma tends to develop multiple brain metastases. Targeted therapy and radiotherapy and other method can be used for the treatment of lung adenocarcinoma with brain metastases of EGFR and/or ALK gene mutation. But for non-mutation multiple brain metastases, radiotherapy is the main treatment method. During whole brain radiotherapy, giving boost radiotherapy to tumors metastases can not only shorten treatment time, but also increase dose of radiotherapy used for brain metastases. Whether such method improves survival chances of patients remains a question. This study aims to investigate the effects of simultaneous integrated boost intensity-modulated radiotherapy (SIB) and sequential boost conformal radiotherapy (CRT) in the treatment of multiple brain metastases from lung adenocarcinoma.
Materials and methodsTop
1. Inclusion criteria
1. Newly diagnosed patients with lung adenocarcinoma, whose pathology is proved to be lung adenocarcinoma through bronchoscopic biopsy and/or lung biopsy, yet having no EGFR and ALK and such gene mutation found in gene detection, diagnosed to have 3 to 5 brain metastases (the diameters of brain metastases were from 0.5 cm to 3 cm) through imaging tests, without other distant metastases, and no history of brainsurgery or radiotherapy; 2. Patients who were in good health without history of chronic diseases or major organs dysfunction, appearing normal in laboratory tests of blood routine, liver and kidney function, electrolytes, blood glucose and electrocardiogram; 3. Patients had no obvious brain metastases, or had mild headache or dizziness, scored 0-2 point in ECOG (Eastern Cooperative Oncology Group); 4. Patients had only brain metastases whose clinical stage were T1-3N0-1M1; 5. Patients who have complete materials and receive regular follow-up as requested. Written informed consent was obtained from all patients, and the institutional research ethics board of Shandongcancer hospital approved this study (No SDTHEC201503048).
2. General clinical data
Forty-two patients with multiple brain metastases from lung adenocarcinoma were analyzed from 2009-01-01 to 2014-12-31 in Shandong cancer hospital affiliated to Shandong University, including 18 male patients and 24 female patients; all pathological types are adenocarcinoma, including 4 high-differentiated cases, 19 moderate-differentiated cases and 19 low-differentiated cases. Table 1 showed the general condition of 42 patients.
| Items | Whole group | SIB group1 | CRT group2 | ρ value3 | |
| Gender | Male | 18 | 11 | 7 | 0.929 |
| Female | 24 | 15 | 9 | ||
| Age (years) | Mean | 57.40 ± 10.11 | 57.54 ± 9.63 | 57.19 ± 11.18 | 0.915 |
| Median | 59 | 59 | 59 | ||
| ECOG scores | Mean | 1.31 ± 0.47 | 1.27 ± 0.45 | 1.38 ± 0.50 | 0.484 |
| Median | 1 | 1 | 1 | ||
| Numbers of brain metastases | Mean | 3.76 ± 0.850 | 3.81 ± 0.89 | 3.69 ± 0.79 | 0.662 |
| Median | 3.5 | 3.5 | 3.5 | ||
| Dose of whole brain radiotherapy (Gy) | Mean | 40.33 ± 4.11 | 40.75 ± 3.63 | 39.64 ± 4.83 | 0.405 |
| Median | 40 | 40 | 40 | ||
| Dose of brain metastases radiotherapy (Gy) | Mean | 55.29 ± 3.83 | 53.99 ± 3.44 | 57.39 ± 3.56 | 0.004 |
| Median | 55 | 55 | 60 | ||
| Times of radiotherapy | Mean | 24.00 ± 3.87 | 22.08 ± 2.45 | 27.12 ± 3.76 | 0.000 |
| Median | 23 | 22 | 30 |
1SIB, Simultaneous integrated boost intensity modulated radiotharapy; 2CRT, Swquential boost conformal radiotherapy; 3ρ values refer to the comparison between SIB group and CRT group.
3. Treatment protocol
The general treatment protocols included radiotherapy and chemotherapy. First-line chemotherapy were included docetaxel or pemetrexed combined with platinum. Radiotherapy included thoracic radiotherapy and brain radiotherapy.
1. Radiotherapy of brain metastases
Radiotherapy positioning and definition of target volume: The patient lay supine on the head immobilized device with thermoplastic mask being fixed; enhanced CT 3 mm-layer-thick sequential scan, and enhanced MRI scan through the same immobilized method; transmitscanned images to the Varian Eclipse planning system workstations. To contour the intracranial metastatic lesions defined as GTV (gross tumor volume, GTV) at the fusion image of enhanced CT and MRI, and to contour the whole brain defined as CTV (clinical target volume, CTV). To expand GTV and CTV by 10 mm and 5 mm defined as PTV-GTV (planning target volume, PTV) and PTV-CTV, respectively.
Radiotherapy plan: Simultaneous integrated boost intensity-modulated radiotherapy group (SIB): prescription dose of PTV-CTV was 1.8 Gy per time, and prescription dose of PTV-GTV was 2.5 Gy per time, 22 times altogether; prescription dose of whole brain was 39.6 Gy, and intracranial metastases was 55.0 Gy (biological equivalent dose was approximately 68 Gy). Radiotherapy would be reset after about 15 times treatment, GTV would be recontoured and the radiotherapy plan would be replaned. Among twenty-six cases in this group, radiotherapywas not carried out 22 times in three cases.
Sequential boost conformal radiotherapy group (CRT): Prescription dosages of PTV-CTV and PTV-GTV are both 2.0 Gy per time. The radiotherapy plan would be replaned after about 20 times treatment and GTV would be recontoured, and the prescription dose of whole brain was 40.0 Gy and intracranial metastases was 60.0 Gy. Among sixteen cases in this group, radiotherapies of two cases were not 30 times. The specific radiation dose and times were shown in Table 1.
The ranges of PTV-CTV total radiation doses used for SIB and CRT groups were 36.0Gy-41.4Gy and 36Gy-40Gy. The ranges of PTV-GTV total radiation doses used for SIB and CRT groups were 50Gy-55Gy and 46Gy-60Gy.
2. The general principles of chemoradiotherapy on primary lung lesions
All patients were given systemic chemotherapy during radiotherapy of whole brain and intracranial metastases. Chemotherapy regimens included docetaxel or pemetrexed combined with platinum. After the brain radiotherapy, primary lung tumors (and/or metastatic lymph node) were given radiotherapy. According to the size of the lesions and thedose of normal tissue tolerance, patients received palliative or radical radiotherapy, and sequential or concurrent chemotherapy. The primary lesions of lung and metastaticlymph node were received radiation 28 to 30 times and the prescription dose was 56-60 Gy.
All the patients received 4 to 6 cycles of chemotherapy and radiotherapy in primary lesions of lung and metastatic lymph node, and 24 cases had concurrent chemoradiotherapy and 18 cases had sequential chemoradiotherapy. All the patients received at least 4 cycles of chemotherapy (docetaxel or pemetrexed combined with platinum).
According to patients' Karnofsky score, blood routine, liver and kidney function, etc., combining with the wishes of patients, we recommended chemoradiotherapy and following with single-agent maintenance chemotherapy.
After radiotherapy and chemotherapy, 31 patients received maintenance chemotherapy, and 15 patients received pemetrexed maintenance chemotherapy (PMC), and 16 patients received docetaxel maintenance chemotherapy (DMC); 11 patients received no maintenance chemotherapy (NMC). The cycles of maintenance chemotherapy were from 2 to 13 cycles, the mean was 7.16 ± 3.46 cycles and the median was 7 cycles.
3. Symptomatic and supportive treatment
During radiotherapy and chemotherapy, patients received steroid hormone dexamethasone 5-10 mg/day and mannitol 250-500 ml/day according to their intracranial pressure symptoms and signs and so on.
4. Follow-up
All patients were given reexamination of enhanced chest and upper abdominal CT and cerebral MRI to evaluate the effect of treatment after one month radiotherapy of brain metastases and primary lung tumors (and/or metastatic lymph nodes). All reexaminations were regularly onceevery three months after treatment of two years, and onceevery six months after treatment of 3-5 years, once every year after treatment of five years. If patients had any discomfort or changes, they could see doctors immediately. Each time of reexamination could be recorded of the patient's symptoms, signs, ECOG score, imaging examination results, adverse reactions of radiation and chemotherapy, such as nerve function change. Reexaminations were including routine blood test, liver and kidney function, electrolytes, glucose, tumor biomarkers and other laboratory examination; enhanced chest and upper abdominal CT, brain MRI, and memory scale evaluation memory.
5. Adverse effect assessment
Radiation Therapy Oncology Group (RTOG) acute and advanced radiation injury grading was used for the adverse effect assessment of radiotherapy, and Common Terminology Criteria for Adverse Events (CTCAE) v4.0 criteria for chemotherapy.
6. Endpoints
The primary endpoints were: overall survival (OS), treatment response, causes of death and adverse effects. Treatment response was defined as no serious symptom or slight symptoms after treatment, no presence of intolerance, no need for pharmacological treatment, lesions disappeared, diminution or stabilization according to original imaging. Treatment failure referred to no improvement or worsening in symptoms, development of lesions presence of or new lesions according to original imaging.
7. Statistical methods
SPSS17.0 was used for Kaplan-Meier survival analysis, Logrank test and multiple factor analysis of prognosis. χ²-Test was used for numeration data and ttest for measurement data. ρ < 0.05 was considered as significant difference. Multiple factors Cox regression analysis was used Wald-Test, and RR was the relative risk.
ResultsTop
1. The treatments of SIB and PMC improved survival time
Contrast-enhanced brain MRI was given one month after treatment for efficacy evaluation, which revealed 3 cases for complete response, 32 for partial response, 5 for stable disease and 2 for disease progression. The response rate was 95.2%. The intracranial and extracranial control rate was 92.8% and 88.1% respectively. The 1-year, 3-year, and 5-year survival rates were 92.8%, 42.8% and 4.8% respectively. The 3-year survival rates were 53.8% and 25.0% in SIB and CRT groups respectively (χ² = 3.365, ρ = 0.067). The median survival time was 36 and 29 months respectively in SIB and CRT groups. The result was better in SIB group than that in CRT group (χ² = 5.714, ρ = 0.022) (Figure 1 and Table 2).
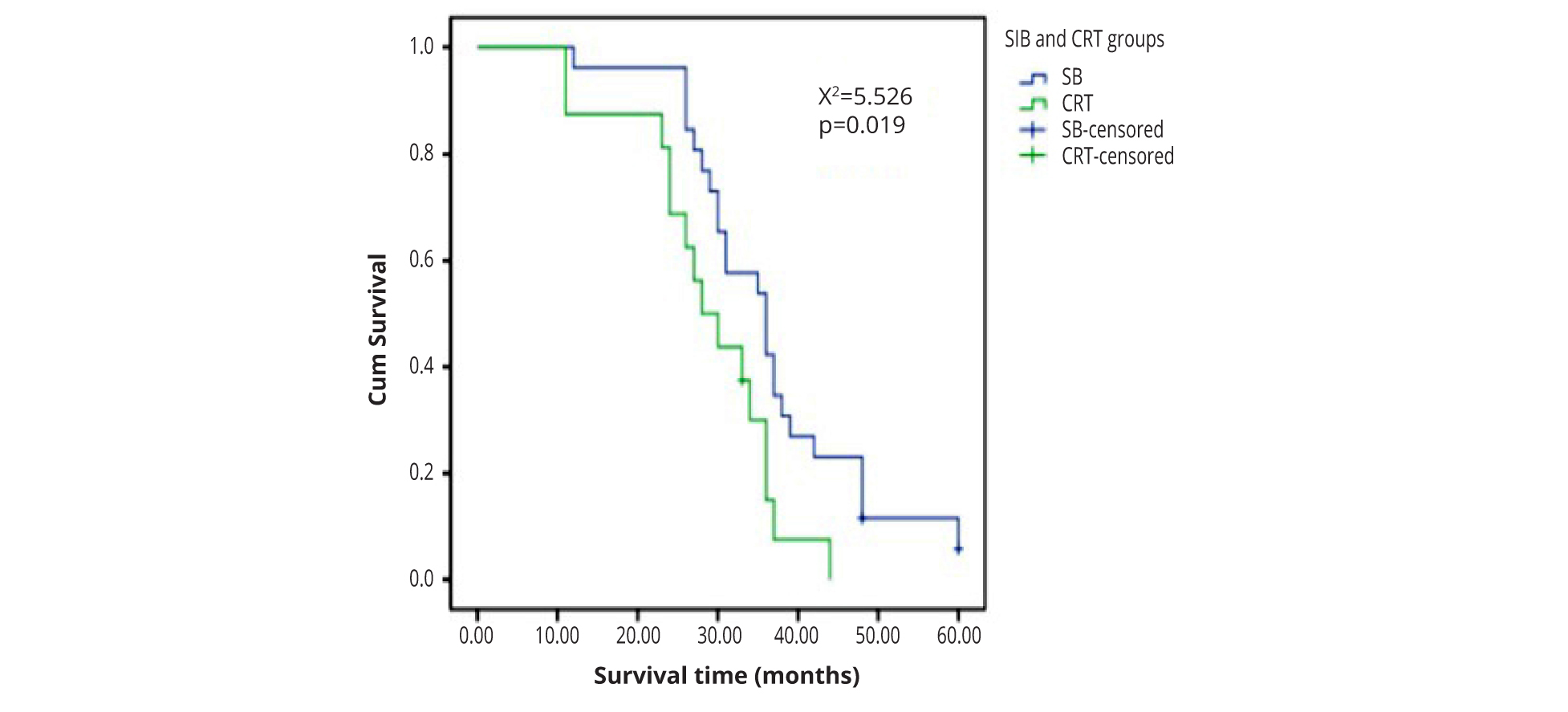
| Items/Groups | Whole group | SIB group1 | CRT group2 | ρ value |
| Cases | 42 | 26 | 16 | |
| Mean survival time (months) | 33.36 ± 10.77 | 36.31 ± 10.90 | 28.56 ± 8.91 | 0.022 |
| Median survival time (months) | 33 | 36 | 29 | 0.019 |
1SIB, Simultaneous integrated boost intensity modulated radiotherapy; 2CRT, Sequential boost conformal radiotherapy.
The median survival time was 36, 33.5 and 28 months respectively in PMC, DMC and NMC. The result showed that PMC was better than DMC or NMC (χ² = 9.852, ρ = 0.007) (Figure 2 and Table 3).
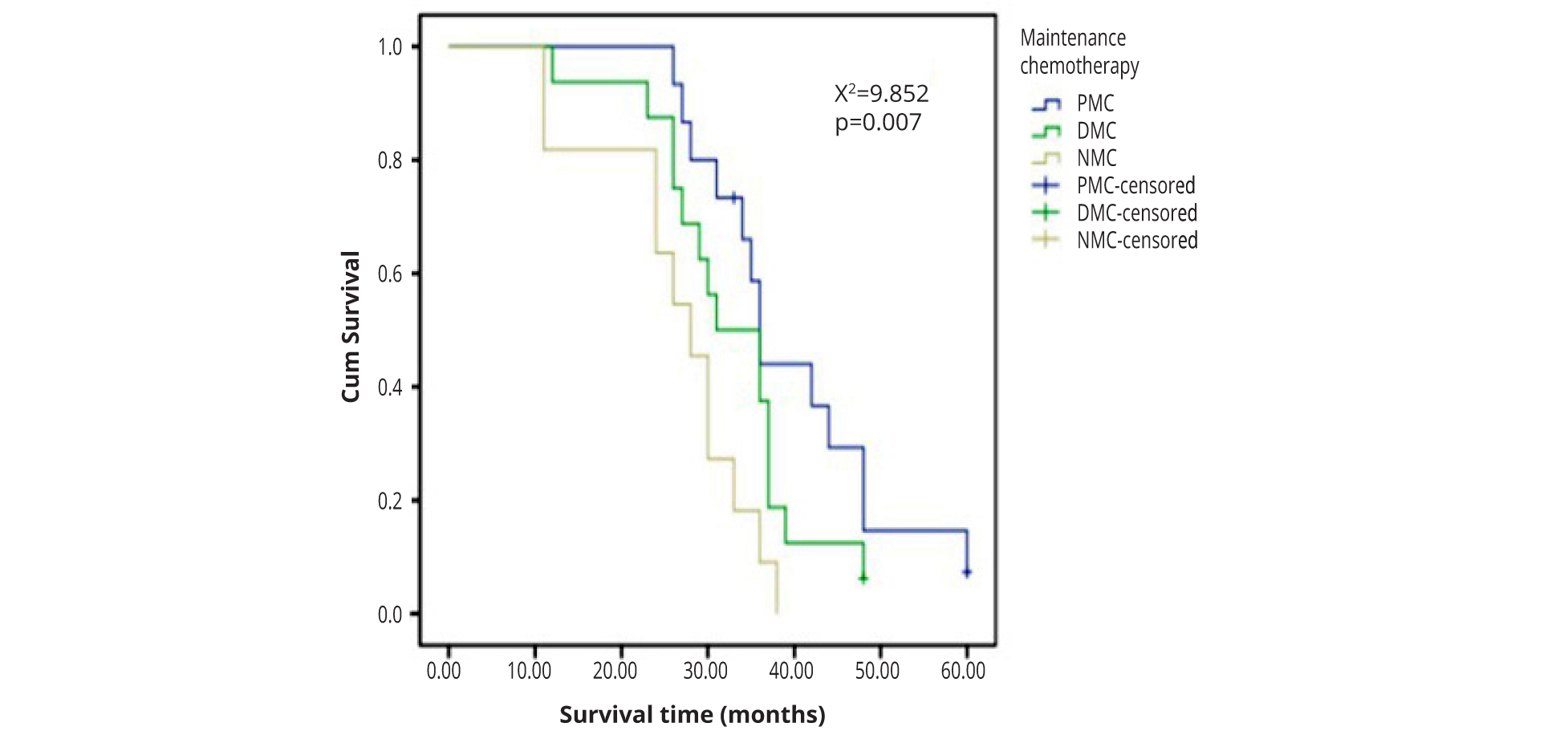
| Items/Groups | Whole group | PMC1 | DMC2 | NMC3 | ρ value |
| Cases | 42 | 26 | 16 | 11 | |
| Mean survival time (months) | 33.36 ± 10.77 | 39.20 ± 10.92 | 32.62 ± 9.14 | 26.45 ± 8.86 | 0.008 |
| Median survival time (months) | 33 | 36 | 33.5 | 28 | 0.007 |
1PMC,Pemetrexed maintenance chemotherapy; 2DMC,Docetaxel maintenance chemotherapy; 3NMC,No maintenance chemotherapy.
2. The factors associated with survival time
The multiple- factor analysis showed that the risk factors related with survival were ECOG scores, radiotherapy dose and times for brain metastases, pemetrexed maintenance chemotherapy and cycles of maintenance chemotherapy. Age, gender, radiotherapy dose for whole brain and pathological grading had no significant influence on survival (Table 4).
| Factors | β | Wald value | RR | 95% CI | p value |
| Gender | 0.077 | 0.12 | 0.899 | 0.642 - 3.263 | 0.729 |
| Age | 0.002 | 0.042 | 1.002 | 0.961 - 1.028 | 0.838 |
| Pathological grading | 0.441 | 1.366 | 1.685 | 3.653 - 10.059 | 0.501 |
| Numbers of brain metastases | 0.126 | 0.353 | 0.882 | 0.580 - 1.339 | 0.552 |
| Dose of whole brain radiotherapy | 0.043 | 2.546 | 1.043 | 0.829 - 0.991 | 0.111 |
| ECOG scores | 1.802 | 48.629 | 6.062 | 0.701 - 4.189 | 0.000 |
| Dose of brain metastases radiotherapy | 0.129 | 29.217 | 0.879 | 0.735 - 0.975 | 0.000 |
| Times of radiotherapy | -0.13 | 4.866 | 1.139 | 1.015 - 1.278 | 0.027 |
| Maintenance chemotherapy | 0.684 | 3.231 | 0.505 | 0.240 - 1.064 | 0.041 |
| Cycles of maintenance chemotherapy | 0.398 | 7.229 | 0.671 | 0.556 - 0.810 | 0.000 |
3. The adverse reactions related to treatment
Ten cases (23.8%) had acute radiation-induced central nervous system reaction with 7 cases of Grade 1 and 3 of Grade 2. The main manifestations were symptoms of acute radiation-induced hydrocephalus, which was improved after mannitol and dexamethasone were given for symptomatic treatment. There were no serious toxicities including cerebral necrosis during brain chemoradiotherapy. There were 15 cases (35.7%) of radiation-induced pneumonia, including 6 cases of Grade 1, 5 of Grade 2, 2 of Grade 3 and 2 of Grade 4. Improvement was seen in 12 cases after treatment, and the other 3 cases died.
4. The main causes of death of patients
All the patients were followed up till December 31, 2016 or till death. 39 in 42 cases (92.8%) died, and the other 3 still survived. 33 cases (84.6%) died of lung cancer progression, among whom 9 (23.1%) died of intracranial metastasis progression and 24 (61.5%) of extracranial metastasis progression. 3 (7.7%) cases died of radiation-induced pneumonia, 2 (5.1%) of myocardial infarction and 1 (2.6%) of traffic accident.
In SIB group, 5 died of intracranial progression, 16 died of extracranial progression, 2 died of myocardial infarction and 1 died of traffic accident, and 2 survived. In CRT group, 4 died of intracranial progression, nine died of extracranial progression, two died of radiation pneumonitis, and one survived. There was no difference on causes of death between SIB and CRT groups (χ² = 0.688, ρ = 0.396).
5. Hypomnesia caused by radiotherapy
According to minimum mental state examination (MMSE), 34 (81.0%) cases presented with hypomnesia [7]. Among the 27 patients above 55 years old, 25 (92.6%) had presented with hypomnesia and the other 2 (7.4%) had no hypomnesia. Among the 15 patients below 55 years old, 9 (60.0%) presented with hypomnesia, and the other 6 (40.0%) had no hypomnesia. There was significant different between those above 55 years old and those not older than 55 years old (χ² = 6.643, ρ = 0.016).
DiscussionTop
The brain is one of the most common metastatic organ sites for lung cancer [1], including single and multiple brain metastasis, the latter is more common, accounting for about 70% [8-10]. There are more therapies for single brain metastasis, including stereotactic radiosurgery (SRS) and stereotactic hypofractionated-radiotherapy (HFRT). But for multiple brain metastasis, the above two therapies are not suitable and the main therapy is conventional radiotherapy, especially for brain metastases without EGFR or ALK gene mutation.
It is recommended by National Comprehensive Cancer Network (NCCN) that radiotherapy in whole brain 30Gy/10 times is the standard therapy for multiple brain metastases no less than 10. But for three to five brain metastases, there is no standard radiotherapy. Our retrospective study revealed that the overall response rate of simultaneous SIB and CRT in whole brain and brain metastases was 95.2% in the 42 patients. The intracranial and extracranial one year control rate was 92.8% and 88.1% respectively. The 1-, 3-, and 5-year survival rates of the whole group were 92.8%, 23.8% and 4.8%, respectively. It is indicated that simultaneous SIB and CRT has satisfactory efficacy and is an acceptable therapy. Totally, 39 in 42 patients died, among whom 33 (84.6%) died of lung cancer progression, including 9 (23.1%) due to intracranial metastasis progression and 24 (61.5%) due to extracranial metastasis progression. Therefore, radiotherapy in the whole brain is effective for controlling intracranial metastasis progression.
The median survival time in SIB and CRT groups was 36 and 29 months respectively, and there was a significant difference between the two groups. According to previous studies [11], whole brain radiotherapy 30Gy/10 times could improve symptoms and extend survival time for those patients with poor general condition and symptomatic multiple metastases. For those with fine general condition, SRS, HFRT or SRS/HFRT in brain metastases plus whole brain radiotherapy both have satisfactory efficacy. The therapy without whole brain radiotherapy has significantly decreased nervous adverse effects, and the therapy without whole brain radiotherapy has significantly more recurrence in intracranial metastases progression. The recent 10-year studies have found that SRS is available for one to four and even less than 10 brain metastases and could improve life quality and survival time [12-14].
Whole brain radiotherapy could reduce intracranial recurrence. Therefore, for those young patients with good general medical condition, weighing the advantages and disadvantages, if the treatment is intended to control intracranial recurrence, SIB in whole brain and intracranial metastases is recommended. Chang et al. found that the 1-year recurrence-free survival time of Stereotactic radiosurgery (SRS) plus WBRT in brain metastases is significantly better than that of single SRS (73% vs 27%, p = 0.0003) [15]. Patchell et al. found that whole brain radiotherapy after surgery in brain metastases is helpful to reduce recurrence rate and improve PFS and life quality [16]. Surgery plus WBRT could significantly reduce intracranial recurrence compared with single surgery (18% vs 70%, p < 0.001) and decrease nerve-related mortality (14% vs 44%, p = 0.003). However, the overall survival between the two groups had no significant difference. Therefore, whole brain radiotherapy could be applied in clinical practice.
The common nervous adverse effect of whole brain radiotherapy is hypomnesia, which is closely related with age. It was found in the present study that, among the 15 patients no younger than 55, 9 (60.0%) had memory loss, and 6 (40.0%) had no serious memory loss. There was significance difference between the group above 55 and no younger than 55 (χ² = 6.643, ρ = 0.016). The main adverse effects of whole brain radiotherapy is nervous damage, and the main manifestations include fatigue, somnolence and recent hypomnesia within 6 months after the treatment, and failing mental powers, poor memories and change in character half a year after the treatment and finally some patients could develop into dementia. It has been found by a long-term follow-up after whole brain radiotherapy the occurrence of dementia could be up to 11% [17]. Therefore, the adverse effects in nervous system are acceptable. The adverse effects influencing patients’ quality of life (QOL) after whole brain radiotherapy were mainly hypomnesia and change in character. Among the 42 patients in the present study, 34 (81.0%) had hypomnesia. Although the rate of hypomnesia was relatively high, it had no significant influence on QOL. The administration of memantine at the time of radiotherapy could reduce cognitive disorder. Memantine is inexpensive and tolerable, and thus could be widely applied [18-21]. In addition, whole brain radiotherapy keeping away from hippocampal synapse could protect the neural stem cells in hippocampal synapse, which is helpful to improve the prognosis, relieve hypomnesia [22, 23] and improve QOL. As it is reported, the metastatic rate of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) to thehippocampal synapse is 2.8% and 18.2% respectively [24]. Gondi et al. reported among 155 cases of NSCLC and 38 cases of SCLC, the rate of metastasis to hippocampal synapse was 3.5% and 7.8% respectively, however, none has hippocampal metastasis [25]. Therefore, the protection of hippocampal synapse is only suitable for preventive whole brain radiotherapy and those patients with relatively fewer brain metastases.
The multiple factor analysis in the present study revealed that the risk factors influencing survival include ECOG score, radiotherapy dose and times of brain metastases. The factors including gender, age, radiotherapy dose in whole brain and pathological grading have no significant influence on survival. The ECOG score is higher, the poorer the prognosis will be, which is to correspondence with the previous reports [26-28]. The radiotherapy dose in intracranial metastases is positively related with the prognosis. In the present study, the dose of SIB was 55 Gy/22 times, and the bioequivalence was about 68 Gy. Thus the median survival time of SIB was significantly longer than that of CRT. It has been reported that whole brain radiotherapy will increase local control rate, which could not transfer into survival benefit. Therefore, whole brain radiotherapy is controversial at present. Especially for those patients with poor general medical condition, best support care has similar efficacy as whole brain radiotherapy [5, 28-30]. It was revealed that radiotherapy times are negatively related with the prognosis. Fewer times of hypofractionation radiotherapy will bring more satisfactory prognosis, which provides basis for SRS or HFRT. But further prospective clinical studies are needed.
This study found that maintenance chemotherapy could improve survival time, and the numbers of C cycles of maintenance chemotherapy were positively related to survival time as reported in the literature [31-34]. The continuation of pemetrexed maintenance therapy was well-tolerated and offers superior OS compared with docetaxel [35], further demonstrating that it is an efficacious treatment strategy for patients with advanced nonsquamous NSCLC and good performance status who did not progress during pemetrexed-cisplatin induction therapy.
In a previous study, patients with one to three brain metastases treated with SRS plus WBRT, had a median OS of 7.4 months for SRS plus WBRT [13]. In this study, the median survival time was 36 and 29 months, respectively in SIB and CRT groups, which was much longer than the OS of IIIA and IIIB stage NSCLCpatients in RTOG0617. The reasons were including: 1. The clinical stage was T1-3N0-1M1 and brain-only oligometastases (the diameters of brain metastases were from 0.5 cm to 3 cm) was potentially cure. 2. Patients who were in good health without history of chronic diseases or major organs dysfunction and had no obvious brain metastases, or had mild headache or dizziness, scored 0-2 point in ECOG. 3. Radical chemo radiotherapy was beneficial to improve survival.
ConclusionTop
Above all, continuation maintenance with pemetrexed may be a feasible treatment option for patients with multiple brain metastases from lung adenocarcinoma. SIB is effective and tolerable for whole brain and brain metastases. The specific dose,numbers of times of radiotherapy and how to reduce radiation- induced hypomnesia need further clinical research.
Conflicts of interest
Authors declare no conflicts of interest.
Funding
This work was financially supported by Key Research and Development Project of Shandong Province in 2015 (grant no 2015GSF118027).
ReferencesTop
[1]Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, et al. Incidence of brain metastasis atinitial presentation of lung cancer. Neuro Oncol. 2015; 17(1):122–128.Article Pubmed
[2]Shao Q, Li J, Li F, Wang S, Wang W, et al. Clinical investigation into the initial diagnosis and treatment of 1,168 lung cancer patients. Oncol Lett. 2015; 9(2):563–568.Pubmed
[3]Gu X, Zhao Y, Xu F. Whole brain irradiation and hypo-fractionation radiotherapy for the metastases in non-small cell lung cancer. ZhongguoFei Ai ZaZhi.2016; 19(4):224–229.Article Pubmed
[4]Fidler IJ. The biology of brain metastasis: Challenges for therapy. Cancer J. 2015; 21(4):284–293.Article Pubmed
[5]Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev ClinOncol.2014; 11(4):203–222.Article Pubmed
[6]Gong X, Zhou D, Liang S, Zhou C. Analyses of prognostic factors in cases of non-small cell lung cancer with multiple brain metastases. Onco Targets Ther.2016; 9:977–983.Article Pubmed
[7]Kirkpatrick JP, Wang Z, Sampson JH, McSherry F, Herndon JE, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int J RadiatOncolBiol Phys. 2015; 91(1):100–108.Article Pubmed
[8]Patchell RA. Brain metastases.NeurolClin. 1991; 9(4):817–824.Pubmed
[9]Law A, Karp DD, Dipetrillo T, Daly BT. Emergence of increased cerebral metastasis after high-dose preoperative radiotherapy with chemotherapy in patients with locally advanced non-small cell lung carcinoma. Cancer.2001; 92(1):160–164.Pubmed
[10]Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J ClinOncol.2004; 22(14):2865–2872.Article Pubmed
[11]Shaw MG, Ball DL. Treatment of brain metastases in lung cancer: Strategies to avoid/reduce late complications of whole brain radiation therapy. Curr Treat Options Oncol.2013; 14(4):553–567.Article Pubmed
[12]Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, et al. Stereotactic radiosurgery plus whole-brain radiation therapy Vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA.2006; 295(21):2483–2491.Article Pubmed
[13]Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA.2016; 316(4):401–409.Article Pubmed
[14]Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol.2014; 15:387–395.Article Pubmed
[15]Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol.2009; 10(11):1037–1044.Article Pubmed
[16]Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA.1998; 280(17):1485–1489.Article Pubmed
[17]DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology.1989; 39(6):789–796.Article Pubmed
[18]Dye NB, Gondi V, Mehta MP. Strategies for preservation of memory function in patients with brain metastases. Chin ClinOncol. 2015; 4(2):24.Article Pubmed
[19]Mehta MP, Ahluwalia MS. Whole-brain radiotherapy and stereotactic radiosurgery in brain metastases: What is the evidence Am SocClinOncolEducBook.2015:e99–104.Article Pubmed
[20]Day J, Zienius K, Gehring K, Grosshans D, Taphoorn M, et al. Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation. Cochrane Database Syst Rev. 2014; (12):CD011335.Article Pubmed
[21]Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013; 15(10):1429–1437.Article Pubmed
[22]Tsai PF, Yang CC, Chuang CC, Huang TY, Wu YM, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: A prospective study. RadiatOncol. 2015; 10:253.Article Pubmed
[23]Mahadevan A, Sampson C, LaRosa S, Floyd SR, Wong ET, et al. Dosimetric analysis of the alopecia preventing effect of hippocampus sparing whole brain radiation therapy. RadiatOncol. 2015; 10:245.Article Pubmed
[24]Harth S, Abo-Madyan Y, Zheng L, Siebenlist K, Herskind C, et al. Estimation of intracranial failure risk following hippocampal-sparing whole brain radiotherapy. RadiotherOncol. 2013; 109(1):152–158.Article Pubmed
[25]Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, et al. Hippocampal-sparing whole-brain radiotherapy: A "how-to" technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J RadiatOncolBiol Phys. 2010; 15:78(4):1244–1252.Article Pubmed
[26]Liu S, Qiu B, Chen L, Wang F, Liang Y, et al. Radiotherapy for asymptomatic brain metastasis in epidermal growth factor receptor mutant non-small cell lung cancer without prior tyrosine kinase inhibitors treatment: A retrospective clinical study. RadiatOncol. 2016; 10:118.Article Pubmed
[27]Rades D, Huttenlocher S, Hornung D, Blanck O, Schild SE. Radiosurgery alone versus radiosurgery plus whole-brain irradiation for very few cerebral metastases from lung cancer. BMC Cancer.2014; 14:931.Article Pubmed
[28]Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, et al. Dexamethasone and supportive carewith or without whole brain radiotherapy in treating patients with non-smallcell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet.2016; 388(10055):2004–2014.Article Pubmed
[29]Khan E, Ismail S, Muirhead R. Incidence of symptomatic brain metastasis following radical radiotherapy for non-small cell lung cancer: is there a role for prophylactic cranial irradiation Br J Radiol. 2012; 85(1020):1546–1550.Article Pubmed
[30]Economopoulou P, Mountzios G. Non-small cell lung cancer (NSCLC) andcentral nervous system (CNS) metastases: role of tyrosine kinase inhibitors (TKIs) and evidence in favor or against their use with concurrent cranial radiotherapy. Transl Lung Cancer Res. 2016; 5(6):588–598.Article Pubmed
[31]Shen L, Niu X, Jian H, Xu Y, Yu Y, et al. Assessment of interfering factors and clinical risk associated with discontinuation of pemetrexed maintenance therapy in advanced non-squamous non-small cell lung cancer. Lung Cancer.2017; 111:43–50.Article Pubmed
[32]Scagliotti GV, Gridelli C, de Marinis F, Thomas M, Dediu M, et al. Efficacy and safety of maintenance pemetrexed in patients with advanced nonsquamous non-small cell lung cancer following pemetrexed plus cisplatin induction treatment: A cross-trial comparison of two phase III trials. Lung Cancer.2014; 85(3):408–414.Article Pubmed
[33]Langer CJ, Paz-Ares LG, Wozniak AJ, Gridelli C, de Marinis F, et al. Safety analyses of pemetrexed-cisplatin and pemetrexed maintenance therapies in patients with advanced non-squamous NSCLC: Retrospective analyses from 2 phase III studies. Clin Lung Cancer.2017; 18(5):489–496.Article Pubmed
[34]Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J ClinOncol.2013; 31(23):2895–2902.Article Pubmed
[35]Raez LE, Santos ES, Webb RT, Wade J, Brito RA, et al. A multicenter phase II study of docetaxel, oxaliplatin, and bevacizumab in first-line therapy for unresectable locally advanced or metastatic non-squamous cell histology non-small-cell lung cancer (NSCLC).Cancer Chemother Pharmacol.2013; 72(5):1103–1110.Article Pubmed



