Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 6, Issue 4, August 2018, Pages 25–31
Case reportOpen Access
Colorectal cancer: Ageing, myeloid-derived suppressor cells, and treatment: Report of two cases
- 1 Immunology Division, UNIFESP, São Paulo, SP, Brazil
- 2 Gastroenterology Division, UNIFESP, São Paulo, SP, Brazil
*Corresponding author: Valquiria Bueno, BSc, PhD., Rua Botucatu 862, 4o andar Immunology Division, UNIFESP, São Paulo, SP, ZIP 04023-900, Brazil. Tel.: 55 11 989622943; E-mail: valquiriabueno@hotmail.com
Received 13 June 2018 Revised 19 July 2018 Accepted 27 July 2018 Published 3 August 2018
DOI: http://dx.doi.org/10.14312/2052-4994.2018-4
Copyright: © 2018 Bueno V, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Background: Colorectal cancer (CRC) is the third cause of cancer in the world and age is a risk factor for this disease. Treatments such as surgical resection and adjuvant chemotherapy have increased the mean survival and the recurrence-free survival. However, the decrease in the frequency and function of effector T cells in addition to the increase in the frequency of myeloid-derived suppressor cells that have been reported in older individuals could contribute to the impaired efficacy in immunity against cancer. We aimed to evaluate if in old patients with CRC, the pre-treatment immunological status was correlated with patients’ outcome. Methods: Patients with CRC (n = 2) were submitted to curative surgical resection or curative surgical resection plus capecitabine/oxaliplatine. Blood was collected for the evaluation of immunological status prior to the treatment and correlated with patients’ outcome. Results: Approximately 20 months after surgery the patients presented with recurrence-free survival. Patient 2 (66-years-old) with CRC and peritoneal metastases presented higher frequency and absolute cell number of myeloid-derived suppressor cells than patient 1 (58-years-old) with CRC. Patient 2 presented a low frequency of effector memory CD8 T cells in addition to high accumulation of effector memory re-expressing CD45RA (CD4 and CD8 T cells). Conclusion: Immunological status was correlated with the disease complexity and our results suggest that removing/reducing myeloid-derived suppressor cells helps in the control of cancer progression. Immunological status is a useful, low invasive tool to identify the disease complexity and the results can be used in the future development of personal treatment according to the immunological status of the patient.
Keywords: colorectal cancer; ageing; myeloid-derived suppressor cells; T cells; chemotherapy
IntroductionTop
Ageing is a risk factor for cancer development and coincides with the reduced effectiveness of the immune system function in the ageing population. The ageing process causes changes in the number and function of T cells, which can contribute, toward tumor development since CD8+ cytotoxic T cells (effector memory) are essential for anti-tumor effects. In addition, suppressive cells such as myeloid-derived suppressor cells (MDSC) increase in number and/or become more suppressive in older individuals.
Colorectal cancer (CRC) is the third cause of cancer in the world [1] and the second in Brazil [2]. In older individuals, CRC is the third in incidence in Latin America and Caribe as well as Australia and New Zealand [3, 4].
Because the occult clinical manifestations of CRC, patients are not diagnosed until reaching advanced stages of the disease [5].
In addition, 20% of CRC patients present metastases at diagnosis with survival from less than nine months (no treatment) to approximately 24 months [6]. Curative surgical resection has been available for patients in advanced stage over the last decade and Gulack et al. [7] reported that patients with CRC stage IV and inoperable metastases when submitted to surgical resection of the primary colorectal tumor presented survival benefit compared to chemotherapy/radiotherapy alone.
Chemotherapy in stage IV metastatic CRC disease has been used as palliative treatment improving quality of life and increasing survival in comparison with supportive care. Zacharakis et al. showed that combined chemotherapy (leucovorin modulated 5-fluorouracil (5-FU); 5-FU + oxaliplatin or irinotecan; capecitabine ± bevacizumab or cetuximab) is an independent predictor of survival for metastatic stage IV CRC. Patients relatively fit (low CRP and tolerate therapy combination) presented a more favourable survival outcome. Recently the FDA approved the use of immunotherapy in metastatic CRC patients with MSI-H (mismatch repair genes deficient- dMMR). These tumors presented many mutations and the patients had increased survival after the use of nivolimab and pembrolizumab in a 3rd or a 4th line [9].
In addition to the treatment of choice, the adequate function of the immune system is essential for anticancer responses. Effector memory T cells (TEM) play a fundamental role in this process but can be overcome by tumor immunosuppressive microenvironment [10, 11]. Several mechanisms have been related to the immunosuppression caused by the tumor development such as myeloid-derived suppressor cells (MDSC). MDSC have been extensively investigated in both cancer experimental models and patients since these cells participate in the cell invasion, and metastases, in addition to the suppression of effector functions (T cells) [3, 12].
MDSC are a heterogeneous population of myeloid cells in immature state. In humans they have been characterized as lineage negative (CD3neg, CD19neg, CD56neg) that express CD11b+ and CD33+ but lack/low expression of HLA-DR. MDSC can be further classified as monocytic (CD14+) or granulocytic (CD15+). These cells exert suppressive effects mainly by induction of T regulatory cells, production of reactive oxygen species (ROS), nitric oxide (NO), arginase (Arg-1), and tumor growth factor β (TGF-β) contributing thus for cancer development. MDSC suppressive effects cause impairment in T cell stimulation, in NK cytotoxicity, in dendritic cell function, and promote conversion of CD4+IL-17+ into a regulatory phenotype [13].
MDSC have been found in higher frequency in patients with cancer, including CRC, in comparison with healthy individuals [14-17]. Flow diagram (Figure 1) shows colorectal cancer (CRC) development and patient’s outcome after treatment. In CRC, the increase of MDSCs the in circulation and/or tumor microenvironment has been associated with nodal and distant metastasis [16, 17], inadequate response to chemotherapy [18], radiotherapy [19] and to prevaccination with human tumor associated antigens (advanced colonic adenomas) [20].
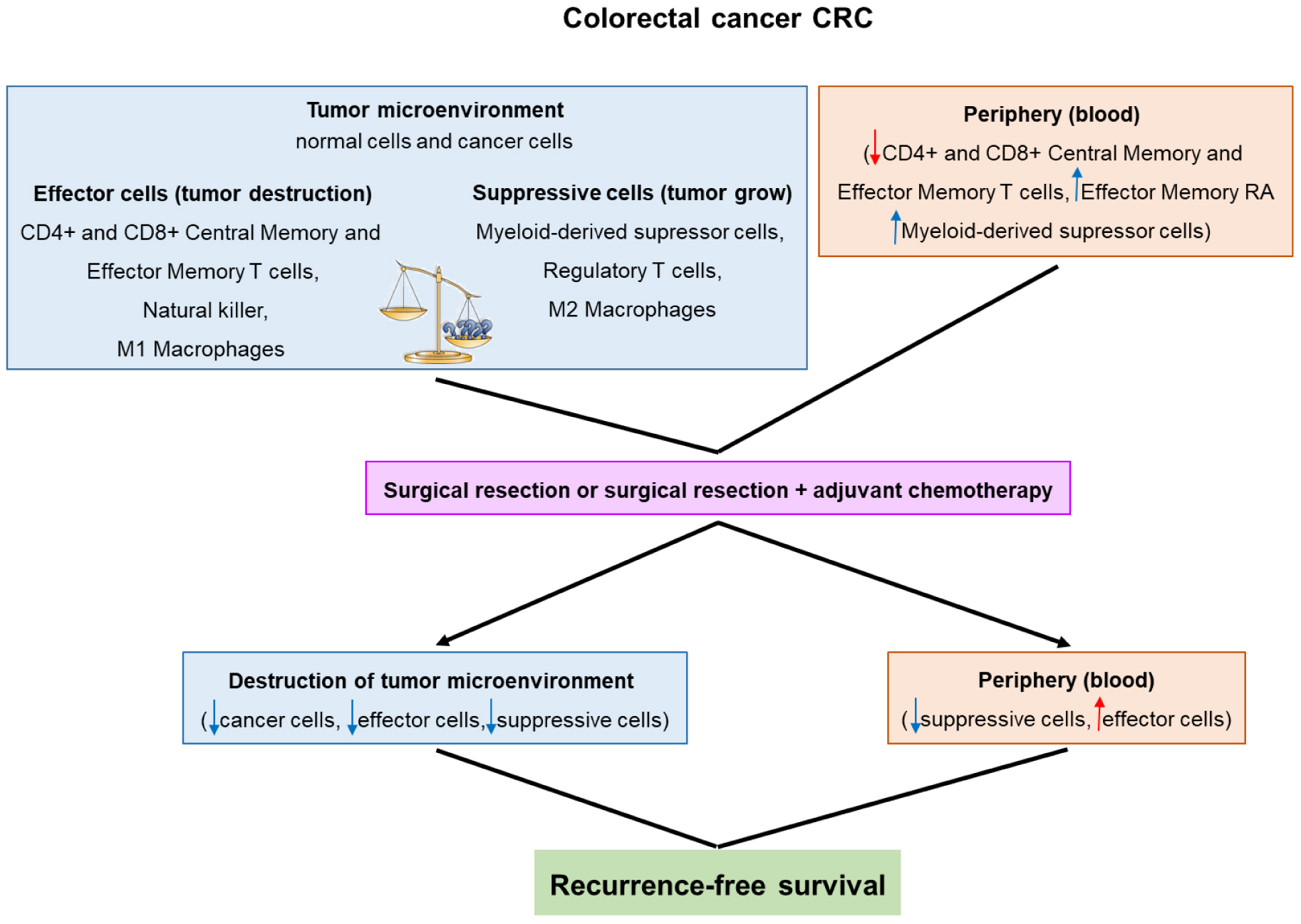
These findings suggest that MDSC accumulation can subvert the outcome of anticancer therapy and thus it has been hypothesized that the immunologic status of the patient can be used as a predictor of treatment response in CRC.
Pagès et al. [21] showed that tumor infiltrating cells evaluated by flow cytometry and presenting a high frequency of CD8+ T cells ranging from early memory (CD45RO+ CCR7-CD28+CD27+) to effector memory (CD45RO+CCR7-CD28-CD27-) were correlated with a less advanced pathological stage, lack of signs of early metastatic invasion, and prolonged survival [10]. The same group observed that the high frequency of CD8+ and memory (CD45RO+) cells in tumor center and invasive margin (immunohistochemistry) was associated with five years of low tumor recurrence (4.8%) and prolonged survival (86.2%) in patients with early-stage CRC [21].
In patients with unresectable metastatic colorectal cancer (mCRC), Tada et al. [18] reported that a combined analysis of MDSC and effector T cells provides the patient’s immune status and correlates with progression-free survival. The group observed that in patients with mCRC (treated with 1st line chemotherapy), the low frequency of MDSC and the high frequency of CD4+ and CD8+ effector memory T cells (TEM) in peripheral blood correlated with higher progression-free survival in 600 days follow-up.
In addition, the immune status could direct the choice for the adequate treatment. Kanterman et al. [15] showed that in advanced CRC there was a high frequency of MDSC and the treatment with FOLFOX (folinic acid, 5-FU, and oxaliplatine) reduced the frequency of circulating MDSC, whereas FOLFIRI (folinic acid, 5-fluorouracil (5-FU), and CPT11) increased the accumulation of MDSC [15]. Using a mouse model and testing monotherapy, it was found that CPT11 alone reduces MDSC sensitivity to apoptosis thus maintaining the immunosuppression. In addition, 5FU + CPT11 promoted cancer progression and short survival that was associated with a dysfunctional immune response [15]. Limagne et al. [22] also found that patients with metastatic colorectal cancer (mCRC) treated with FOLFOX-bevacizumab present a decrease of MDSC (granulocytic phenotype, gMDSC). Interestingly, the reduction in gMDSC occurred mainly in patients with an initial high incidence of this cell subtype suggesting that in the studied patients, gMDSC were more susceptible to chemotherapy-induced cell death. These findings suggest the immune status could be used to choose the better treatment and in the presence of a suppressive microenvironment (high frequency of MDSC) the therapy should avoid agents that contribute to an increase of immunosuppression. The present study reports two cases of CRC, their pre-treatment immune status, and the patients’ outcome.
MethodsTop
Blood was collected from two patients diagnosed with CRC and 2 healthy controls (25 and 52-years-old) at Hospital São Paulo – UNIFESP prior to any treatment. Patient 1 was 58-years-old with stage III (T3N1M0) and patient 2 was 66-years-old with stage IV (T3N1M1) with peritoneal metastasis. A local committee (UNIFESP Ethics Committee 0250-2016) approved the study.
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque density gradient (Amersham Biosciences, Uppsala, Sweden) and centrifugations. Viable cells were counted, adjusted for 1x106/100 µL and stained with monoclonal antibodies to T cell phenotype CD3 APC, CD4 PerCP Cy 5.5, CD8 APC Cy7, CD27 FITC, CD45RA PE (eBioscience, CA, USA). The cells were also stained with monoclonal antibodies to MDSC phenotype CD3 APC, CD19 APC, CD56 APC, HLA-DR APC e-fluor 780, CD33 PerCP Cy5.5, CD11b PE, CD14 PE Cy7, CD15 FITC (eBioscience, CA, USA). After 30 min of incubation with monoclonal antibodies, in the dark and at 4°C, the cells were washed with PBS and centrifuged. Living cells (based on forward and side scatter) were acquired in the FACS Canto II using the DIVA software (Becton Dickinson, USA). Further analyses of FACS data were performed using the 9.3 FLOWJO software (Tree Star, Inc., USA).
Myeloid-derived suppressor cells were characterized as
CD3-CD19-CD56-HLA-DR−/lowCD33+CD11b+CD14+ monocytic
CD3-CD19-CD56-HLA-DR−/lowCD33+CD11b+CD15+ granulocytic
T lymphocytes were characterized as
Naïve: CD3+CD4+CD45RA+CD27+ or CD3+CD8+CD45RA+CD27+ (Naïve)
Central memory: CD3+CD4+CD45RA-CD27+ or CD3+CD8+CD45RA-CD27+ (CM)
Effector memory: CD3+CD4+CD45RA−CD27- or CD3+CD8+CD45RA-CD27- (EM)
Effector memory re-expressing CD45RA: CD3+CD4+CD45RA+CD27- or CD3+CD8+CD45RA+CD27- (EMRA)
ResultsTop
Patient treatment/outcome
1- Patient diagnosed in November 2015 and submitted to tumor resection in April 2016. No recurrence of disease was observed until January 2018 (30 months after diagnosis by colonoscopy and 21 months after surgery).
2- Patient diagnosed in January 2016 and submitted to tumor resection in May 2016. During the surgery, peritoneal metastases was diagnosed. After chemotherapy (CAPOX: capecitabine and oxaliplatine) treatment from June 2016 to November 2017, tumor disease was not diagnosed and chemotherapy was withdraw. Follow-up of 22 months after surgery (March 2018) shows absence of disease by PET CT Scan.
Immunological status (pre-treatment) MDSCs (Figure 2, Table 1) increased in frequency and the absolute cell number in the middle-aged (52-years-old) healthy control and this increase was more vigorous in patients with cancer compared to the young healthly controls. Patient 2 (CRC + peritoneal metastasis) presented a 6.6-fold increase in the frequency and 3.6-fold increase in the absolute cell number of MDSC in comparison with patient 1 (CRC, no metastases). In patient 2, monocytic and granulocytic subtypes of MDSC presented a 4-fold increase in the frequency in comparison with the healthy controls (Figure 2).
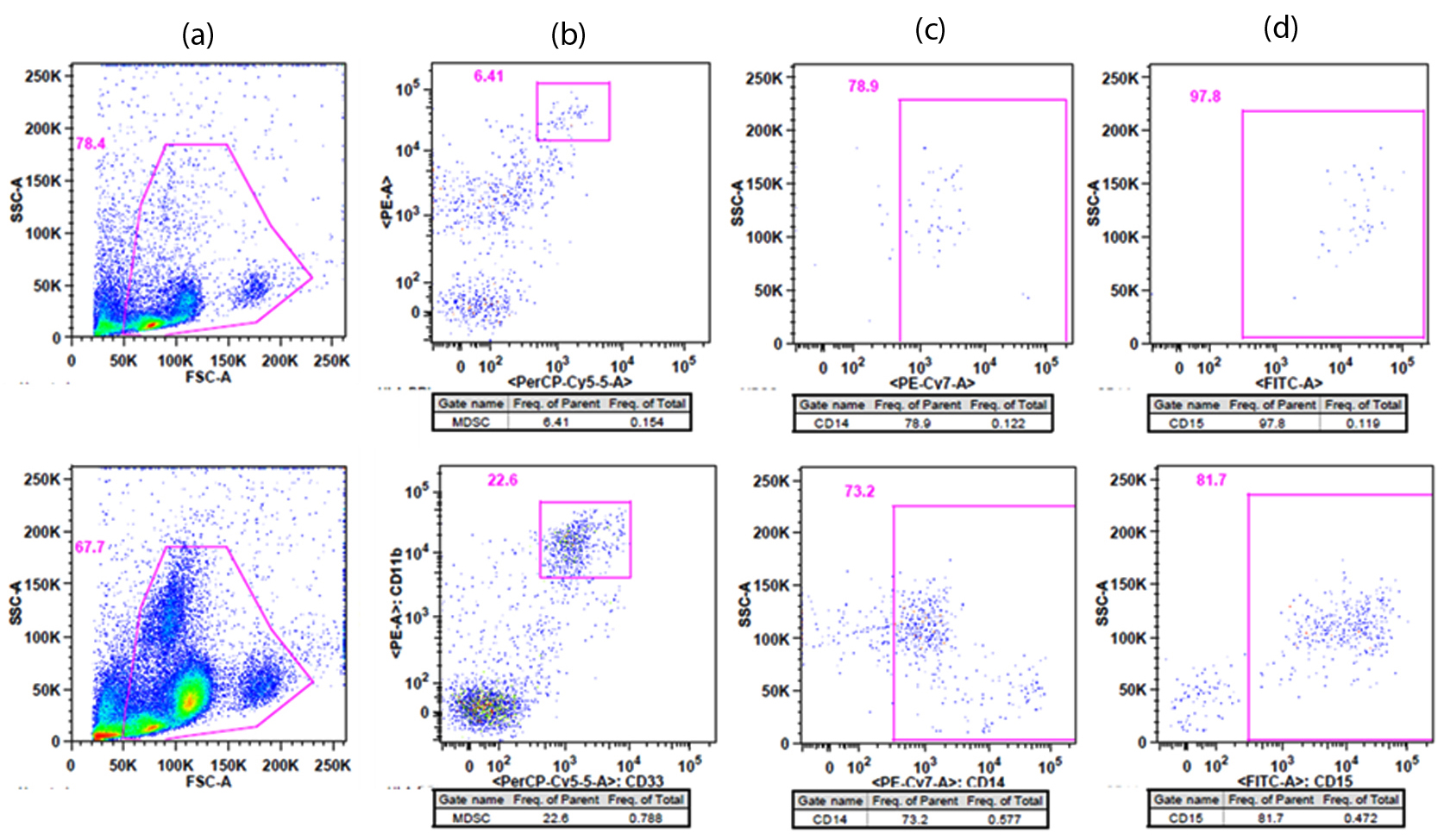
| Markers | Healthy controls | Patients with CRC | ||
| 25 years old | 52 years old | 58 years old | 66 years old | |
| MDSC | 0.05% | 0.15% | 0.12% | 0.79% |
| Absolute Cell number MDSC | 0.039 × 105 | 0.7 × 105 | 1.5 × 105 | 5.35 × 105 |
| CD4+ Naïve | 54% | 17% | 8.9% | 18.3% |
| CD4+ Central Memory - TCM | 29.7% | 57.5% | 44% | 46.8% |
| CD4+ Effector Memory - TEM | 14% | 21.0% | 36.8% | 12.8% |
| CD4+ Effector Memory RA - TEMRA | 2% | 4.4% | 11.0% | 22.1% |
| CD8+ Naïve | 52.7% | 26.1% | 41.5% | 40.4% |
| CD8+ Central Memory - TCM | 22.4% | 51% | 20% | 15% |
| CD8+ Effector Memory - TEM | 11.2% | 12.3% | 6.6% | 3.0% |
| CD8+ Effector Memory RA - TEMRA | 13.2% | 10.6% | 32% | 41.6% |
CD4 Naïve T cells (Figure 3, Table 1) decreased in frequency in middle-aged healthy control and in patients with cancer compared to young healthy control. CD4 central memory T cells increased in frequency with age and in cancer patients. CD4 effector memory T cells increase in frequency with age and in patient 1, whereas it decreased in patient 2. CD4 EMRA T cells increased in frequency with age and in patients with cancer, the frequency was higher in the more complex disease (patient 2).
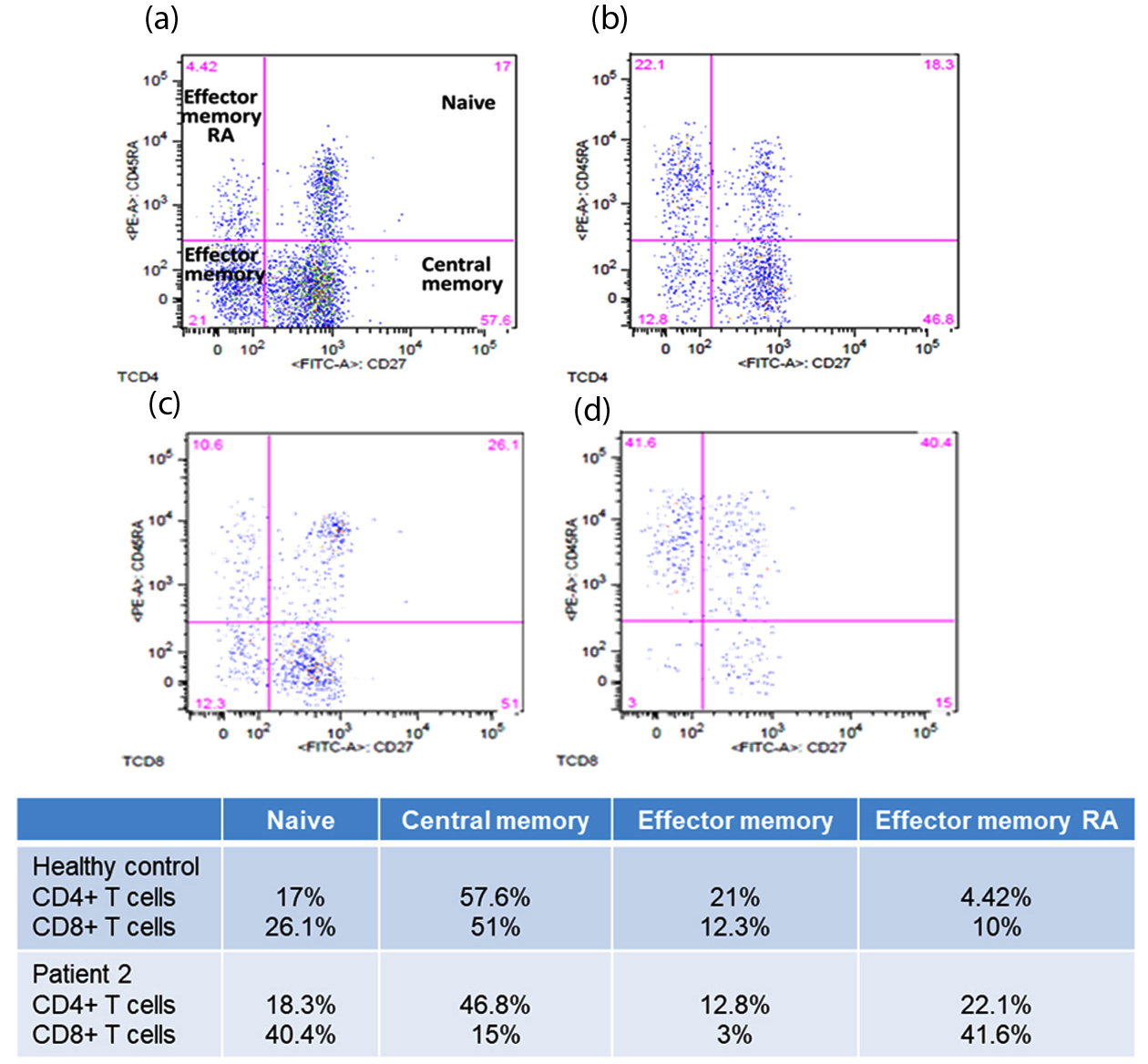
CD8 Effector T (cytotoxic) cells are fundamental for anticancer responses and these cells differentiate from Naïve CD8 T cells. The frequency of Naïve CD8 T cells (Table 1) was higher in healthy young control (52.7%), decreased in middle-aged healthy control, and increased in Patient 1 and 2. However, there was no increase in central memory CD8 T cells and in addition, there was a decrease in effector memory CD8 T cells that could “fight” the disease. On the other hand, there was an accumulation of late differentiated CD8 T cells (TEMRA) that was also correlated with the complexity of the disease (higher in patient 2).
DiscussionTop
Our results of two patients of 58 (curative surgery) and 66-years-old (curative surgery plus CAPOX) showed that both treatments promoted disease-free survival for approximately 20 months of follow-up. Although, most tumors of patients with CRC are resected, the incidence of recurrence is 30-40% during follow-up. Adjuvant chemotherapy in colon cancer stage III are nowadays, the standard of treatment. Fluopyrimidines (5-FU or capecitabine) and oxaliplatine increase the mean survival and the recurrence-free survival [23, 24].
The increase in the number of older patients presenting with CRC has mobilized clinicians to investigate new treatments in this population. Berardi et al. [25] administered 5-FU plus oxaliplatin (FOLFOX) or CPT11 (FOLFIRI) to 29 patients, aged 70 years or older and diagnosed with mCRC. They observed a partial response in approximately 28%, stable disease in 38% and progressive disease in 35%, with a median survival of 21 months. The authors concluded that both regimens were well tolerated and the toxicity was restricted to grade III leukopenia (7%) and grade III diarrhoea [25].
In our cases, CRC was associated with an increase in the frequency and absolute cell number of MDSC (monocytic and granulocytic subtypes), which was more evident in CRC plus peritoneal metastases. In agreement, Toor et al. [26] found an increased percentage of granulocytic MDSC in the circulation and tumor tissue of patients with higher stage and histological grade of cancer. The authors also observed that granulocytic MDSC from the blood of patients produced higher levels of arginase in comparison to healthy donors.
In our study, effector memory CD8 T cells were more affected by CRC and presented a low frequency in the patient with mCRC. This could be related to the suppressive effects of MDSC that prevent T cell differentiation (high MDSC in patient 2). CD4 and CD8 TEMRA accumulation has been associated with ageing and age-related diseases (inflammation). In our cases, there was a higher frequency of these cells in the older patient, which also presented a more complex disease. This patient in a follow-up of 22 months presented recurrence-free survival. We may speculate that the chemotherapy (CAPOX) administered was capable of inducing cell death of the remaining MDSC [15], improving T cell responses, and leading therefore to recurrence-free disease and prolonged survival [25]. Patient 1 had a curative surgery and is disease-free even though this patient presented at the time of diagnosis with an elevated frequency of MDSC, low frequency of CD8 effector memory and an accumulation of TEMRA cells.
Ye et al. [27] observed that patients with CRC presented a higher percentage of CD8 TEMRA in peripheral blood than in tumor infiltrating lymphocytes (TILs). The authors also found that CD8 effector memory cells were expressed in a higher percentage in TILs than in peripheral blood. However, CD8 T cells from peripheral blood presented higher amounts of perforin, a cytotoxic molecule, whereas only 0-13% of CD8 T cells from TILs expressed perforin. These findings suggest that, in tumor tissue, antigen-experienced CD8 effector memory presented a functional tolerant state instead of a cytotoxic function.
Our findings suggest that removing/reducing MDSC from the circulation (surgery, or surgery + chemotherapy) helps in the control of cancer progression.
MDSC, CD8 TEM, and CD4 and CD8 TEMRA measurement in peripheral blood is a useful, low invasive tool to identify the immune status, to direct the treatment, and to estimate disease-free and overall survival in CRC. In agreement, Tada et al. [18] observed that patients with unresectable mCRC treated with FOLFOX or XELOX and presenting high monocytic MDSC, low CD4 and CD8 effector memory T cells expressions had a significantly shorter progression-free survival.
ConclusionTop
The evaluation of MDSC, CD4 and CD8 status prior to treatment is an important strategy particularly for older individuals. Our observations, admittedly limited to two cases, could with evidence from further studies, direct the treatment and optimize the results in addition to preventing the toxicity caused by inadequate chemotherapy.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) financial support (Project 2014/50261-8).
ReferencesTop
[1]Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5):359–386.Article Pubmed
[2]Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância Estimativa 2016: incidência de câncer no Brasil/ Instituto Nacional de Câncer José Alencar Gomes da Silva – Rio de Janeiro: INCA, 2015.
[3]Forones NM, Bueno V. Cancer, Ageing and Immunosenescence. In: The Ageing Immune System and Health. Bueno V., Lord JM, Jackson T (Editors). Switzerland: Springer. 2017:105–124.Article
[4]Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009; 136(3):741–754.Article Pubmed
[5]Gonzalez-Pons M, Cruz-Correa M. Colorectal Cancer Biomarkers: where are we now? Biomed Res Int. 2015; 2015:149014.Article Pubmed
[6]Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008; 134(5):1296–1310.Article Pubmed
[7]Gulack BC, Nussbaum DP, Keenan JE, Ganapathi AM, Sun Z, et al. Surgical resection of the primary tumor in stage iv colorectal cancer without metastasectomy is associated with improved overall survival compared with chemotherapy/radiation therapy alone. Dis Colon Rectum. 2016; 59(4):299–305.Article Pubmed
[8]Zacharakis M, Xyos ID, Lazaris A, Smaro T, Kosmar C, et al. Predictors or survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010; 30(2):653–660.Article Pubmed
[9]Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372(26):2509–2520.Article Pubmed
[10]Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005; 353(25):2654–2666.Article Pubmed
[11]Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012; 12(4):298–306.Article Pubmed
[12]Bueno V, Sant’Anna OA, Lord JM. Ageing and myeloid-derived suppressor cells: possible involvement in immunosenescence and age-related disease. AGE (Dordr). 2014; 36(6):9729.Article Pubmed
[13]Shipp C, Speigl L, Janssen N, Martens A, Pawelec G. A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell Mol Life Sci. 2016; 73:4043–4061.Article Pubmed
[14]OuYang Ly, Wu Xj, Ye SB, Zhang RX, Liao W, et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J Transl Med. 2015; 13:47.Article Pubmed
[15]Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, et al. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014; 74(21):6022–6035.Article Pubmed
[16]Sun HL, Zhou X, Xue YF, Wang K, Shen YF, et al. Increased frequency and clinical significance of myeloid derived suppressor cells in human colorectal carcinoma. World J Gastroenterol. 2012; 18:3303–3309.Article Pubmed
[17]Zhang B, Wang Z, Wu L, Zhang M, Li W, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013; 8:e57114.Article Pubmed
[18]Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, et al. Pretreatment immune status correlates with progression-free survival in chemotherapy-treated metastatic colorectal cancer patients. Cancer Immunol Res. 2016; 4(7):592–599.Article Pubmed
[19]Napolitano M, D’Alterio C, Cardone E, Trotta AM, Pecori B, et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget. 2015; 6(10):8261–8270.Article Pubmed
[20]Kimura T, McKolanis JR, Dzubininski LA, Islam K, Potter DM, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev Res (Phila). 2013; 6(1):18–26.Article Pubmed
[21]Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009; 27(35):5944–5951.Article Pubmed
[22]Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 2016; 76(18):5241–5252.Article Pubmed
[23]André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004; 350(23):2343–2351.Article Pubmed
[24]Haller DG, Tabernero J, Maroun J, de Braud F, Price T, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011; 29(11):1465–1471.Article Pubmed
[25]Berardi R, Saladino T, Mari D, Silva RR, Scartozzi M, et al. Elderly patients with advanced colorectal cancer: Tolerability and activity of chemotherapy. Tumori. 2005; 91(6):463–466.Pubmed
[26]Toor SM, Syed Khaia AS, El Salhat H, Bekdache O, Kanbar J, et al. Increased levels of circulating and tumor-infiltrating granulocytic myeloid cells in colorectal cancer patients. Front Immunol. 2016; 7:560.Article Pubmed
[27]Ye SW, Wang Y, Valmori D, Ayyoub M, Han Y, et al. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major-effector memory subset with low perforin content. J Clin Immunol. 2006; 26(5):447–456.Article Pubmed
[28]Koch M, Beckhove P, Op den Winkel J, Autenrieth D, Wagner P, et al. Tumor infiltration T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg. 2006; 244(6):986–992.Article Pubmed
[29]Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol. 2015; 6(2):208–223.Article Pubmed



