Journal of Cancer Research & Therapy
An International Peer-Reviewed Open Access Journal
ISSN 2052-4994


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Cancer Research & Therapy
Volume 9, Issue 3, September 2021, Pages 20–22
Original researchOpen Access
Vulva and lower limb cancer: Results of inguinal lymph node staging on 81 cases
-
Sidy KA1,*
 , Mamadou M Dieng1, Jaafar Thiam1 , Souleymane Dieng1 , Adja Coumba Diallo1 , Doudou Diouf1 , and
Ahmadou Dem1
, Mamadou M Dieng1, Jaafar Thiam1 , Souleymane Dieng1 , Adja Coumba Diallo1 , Doudou Diouf1 , and
Ahmadou Dem1
- 1 Cheikh Anta Diop University, Joliot Curie Cancer Institute Dakar, Aristide LeDAntec Hospital, Avenue Pasteur, Dakar, Senegal
*Corresponding author: Sidy KA, Surgical Oncologist, Cheikh Anta Diop University, Joliot Curie Cancer Institute Dakar, Aristide LeDAntec Hospital, Avenue Pasteur, Dakar, Senegal. E-mail: sidy.ka@ucad.edu.sn
Received 24 May 2021 Revised 2 August 2021 Accepted 16 August 2021 Published 27 August 2021
DOI: http://dx.doi.org/10.14312/2052-4994.2021-3
Copyright: © 2021 Sidy KA, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Background: The objective of this work completed at the Cancer Institute in Dakar is to report the results of inguinal lymph node dissection in cancers of the lower limb and of the lower genital tract. Methods: This is a retrospective study over a 10-year period. The parameters being studied are histological type, lymph node involvement, postoperative morbidity, recurrence, and survival. Results: 81 patients received surgery over a period of 7 years. The average age of our patients was 61. The sex ratio is 0.74 with 34 men and 47 women. There were 70 cases of cancer of the lower limbs (86%) and 11 cases of cancer of the vulva (14%). The most common histological type was squamous cell carcinoma (SCC) with 41 cases (51%). Clinical inguinal involvement was noted in 58 patients (72.5%) with palpable lymph nodes. All vulvar cancer patients developed histologically positive nodes. Melanoma patients were more susceptible to developing positive nodes. In sarcoma there were more matches between clinical and histological positive nodes. No vascular and nerve damage was reported. The average length of hospitalization was 5 days, with 3 days being the shortest stay, and 40 days the longest stay. Local complications consisted of suture releases in 9 cases, and 6 surgical necrosis of the wound. A seroma was found with an average duration of 35 days in 69 patients (85%). Postoperative deaths occurred in 5 cases (6%), 1 after a renal failure, 1 due to thromboembolic disease, 1 due to sepsis, and 2 deaths occurred after patients experienced respiratory distress. Conclusion: After five years of follow-up care, no patient presented chronic sequelae after inguinal dissection, 7 patients (8.75%) had local recurrence, and 4 patients (7.7%) had lymph node metastases. We recorded 33 cancer-related deaths (41%). Chronic complications, including lymphedema are underestimated and require better assessment methods for prevention and treatment.
Keywords: inguinal; limb; vulvar; node; dissection
IntroductionTop
Lymph node dissection is done for the staging and removal of metastatic node involvement. Most inguinal nodes are primary lymphoma or secondary localization of skin cancers, soft tissue sarcomas, and osteosarcomas of the lower limb or lower genital tract organs [1].
After inguinal lymph node dissection, many complications can occur [2]. More than 16% of women with vulvar cancer will develop lymph node involvement [3]. The objective of this work is to report the results of inguinal lymph node dissection of lower genital tract and lower limb cancers conducted at the Joliot Curie Cancer Institute.
MethodsTop
We performed a retrospective study over a 10-year period that included patients over 15 years old. We selected all the patients who had surgery on their lower limbs or lower genital tract such as the vulva and vagina. We found no case of vaginal or penile cancer. The parameters studied were the site of the lower limb or the vulva (the parameters studied were cancer sites), histological type, lymph node involvement, operative morbidity, recurrence, and survival. The settings have been listed on Excel (We used Excel to codify the data). In this analytical study, we report the incidence of different parameters without using a comparative study with a control group, because in our practice we systematically perform inguinal dissection.
ResultsTop
There were 81 patients operated on over a period of 7 years. The average age of our patients was 61 years. The sex ratio was 0.74, with 34 men and 47 women. The limb localization accounted for 70 patients (86%), while 11 patients (14%) presented vulvar carcinoma.
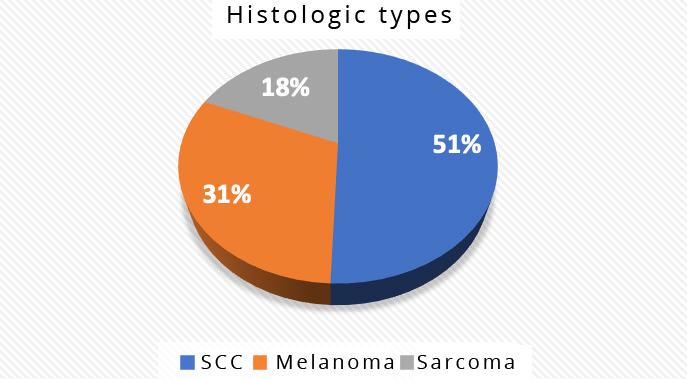
The most common histological type was squamous cell carcinoma (SCC) with 41 cases (51%), followed by acral melanoma in 25 cases (31%), and sarcomas in 15 cases (18.5%). All lower genital tract cancers were SCC, and we had 11 such cases (Figure 1).
Clinical inguinal involvement with palpable lymph nodes was noted in 58 patients (72.5%). Among those 58 patients, 15 had malignant fungi which are clusters of ulcerated lymph nodes fistulized on the skin. All patients with vulvar cancer underwent complete vulvectomy because they presented with advanced stages and histologically positive nodes. Lymph nodes were affected clinically in limb cancer cases, and on histology in cases of sarcomas (Table 1).
Histologic type |
Non palpable nodes |
Palpable nodes |
| Melanoma | 6(24%) |
19(76%) |
| SCC | 9(30%) |
21(70%) |
| Sarcoma | 3(20%) |
12(80%) |
| Total | 18(25,7%) |
52(74,3%) |
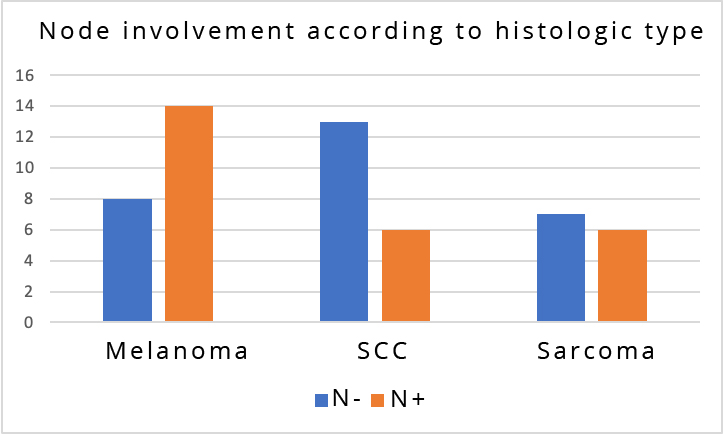
Histological results of node examination were obtained in 32 of 58 patients (55%). While patients with melanoma were more susceptible to develop positive nodes, in patients with sarcoma there were more matches between clinical and histological positive nodes (Figure 2). No vascular or nerve damage was reported. The average length of hospitalization was 5 days. Local complications consisted of suture releases in 9 cases, and 6 surgical necrosis of the wound. A seroma was found with an average duration of 35 days in 69 patients (85%). Postoperative death occurred in 5 cases (6%), 1 in a context of renal failure, 1 in thromboembolic disease, 1 in sepsis, and 2 in a context of respiratory distress. After five years of follow-up, no patient presented with chronic sequelae after inguinal dissection, 7 patients (8.75%) had local recurrence, and 4 patients (7.7%) had lymph node metastases. We recorded 33 cancer-related deaths (41%).
DiscussionTop
The average age of onset of cancers of the lower limbs is 50 years, and 60 years for vulvar carcinoma [1, 3]. The mean age of inguinal dissection according to the authors depends on the histological type, and it ranges from 20 to 60 years for sarcomas to more than 70 years for SCC [4, 5]. The sex ratio varies according to the histological type with a general tendency in favor of men. However, it is shown that women are more likely to developpe squamous cell carcinoma in lower extremities and vulvar carcinoma in advanced ages [6, 7]. Clinical lymph node involvement varies according to the histological type. Melanoma is more likely to involve the lymph nodes than other histological types [8]. It is common to find inguinal lymph nodes in large, inflammatory, undifferentiated, and high-grade sarcomas [9]. In vulvar and lower vaginal carcinomas, clinical lymph nodes are found in 25% of cases [3]. In our study, because of locally advanced cases, all patients underwent complete vulvectomy and were node positive. Inguinal dissection in these cases is strongly recommended [3, 10].
The lymph node ratio is correlated with tumor size and stage. It appears to be a consistent and independent prognostic parameter for progressive free survival (PFS) and overall survival (OS), and it allows patients to be stratified into three distinct risk groups. In survival analyzes, the lymph node ration (LNR) is more indicative than the lymph node status and the number of positive nodes [11].
Histological lymph node involvement is also variable depending on the histological type [12]. Capsular invasion and the number of lymph nodes affected are important prognostic factors, particularly in melanoma. It remains unclear whether the inguinal clearance limited to the groin or extended to the iliac area is dependent on whether the Cloquet's node is affected or not [5, 7]. Squamous cell carcinoma and sarcomas of any stage are unlikely to affect the inguinal lymph nodes [9]. The morbidity of inguinal lymph node dissection is close to 70%, and there is considerable variation between authors [12]. There is a high frequency of postoperative complications such as ruptured sutures, skin necrosis, seroma and early lymphedema. Oblique incisions are less deleterious than vertical incisions, while deep dissection is a better [13]. We had no weakening of the groin, which sometimes can be responsible for inguinofemoral hernia [14]. Bilateral inguinofemoral lymphadenectomy has now been replaced by selective inguinal lymphadenectomy depending on the choice of unilateral dissection in unilateral and non-medial vulvar lesions, and the use of lymph node mapping to minimize perioperative complications [15]. In case of melanoma surgery, morbidity is shown to be high, and the use of laparoscopy-assisted inguinal dissection seems to decrease complications [16]. Mortality directly related to inguinal dissection as well as postoperative mortality is very low, and is linked to co-morbidities and the underlying cancer [17]. Recurrences are mainly seen at the site of the primary cancer. They are very rare at the inguinal site, with the exception of malignant fungi [18]. Although rare in our series, the most common chronic complication is lymphedema. It is more common after radiotherapy to the limb or groin [12]. Many methods are increasingly used for lymphedema evaluation, prevention and treatment. Such methods include endoscopic and micro-invasive surgical techniques, drainage, dissection and physiotherapy. As with all comparisons between open and keyhole surgery, the focus should be on improving the open technique to its limit [3, 19]. Whether prophylactic are performed after sentinel node biopsy, inguinal lymph node dissection has not shown any significant benefit in terms of overall survival. However, it can prevent crippling inguinal disease [20].
ConclusionTop
Clinical and histological inguinal lymph node involvement is more frequent in our practice than in the literature pertaining to lower limbs and genital tract. This justifies a maximalist therapeutic attitude to avoid inguinal recurrence. Chronic complications, including lymphedema are underestimated and require better assessment methods for prevention and treatment. It is important to educate the general public in order to help with the early diagnoses and screenings of cancers so as to optimize the results of their treatments.
Ethics approval and consent to participate
This article follows our institution’s ethics committee rules.
Conflicts of interest
There are no competing interests.
ReferencesTop
[1]Enes H, Balak D. Images in clinical medicine. Metastatic inguinal lymphadenopathy. N Engl J Med. 2012; 366(16):1526.Article Pubmed
[2]Tobias-Machado M. Video endoscopic inguinal lymphadenectomy (VEIL): is a new standard ready to be accepted BJU Int. 2017; 119(4):504–505.Article Pubmed
[3]Huang J, Yu N, Wang X, Long X. Incidence of lower limb lymphedema after vulvar cancer: A systematic review and meta-analysis. Medicine. 2017; 96:46(e8722).Article Pubmed
[4]Alam M, Ratner D. Cutaneous squamous cell carcinoma. N Engl J Med. 2001; 344(13):975–983.Article Pubmed
[5]Kibarian MA, Hruza GJ. Nonmelanoma skin cancer. Risks, treatment options, and tips on prevention. Postgrad Med. 1995; 98(6):39–40.Article Pubmed
[6]Kim Y, Feng BSJ, Su KA, Asgari MM. Sex based differences in the anatomic distribution of cutaneous squamous cell carcinoma. Int J Wom Derm. 2020; 6(4):286–289.Article Pubmed
[7]Weinberg D, Gomez-Martinez RA. Vulvar Cancer. Obstet Gynecol Clin North Am. 2019; 46(1):125–135.Article Pubmed
[8]Henderson MA, Gyorki D, Burmeister BH, Ainslie J, Fisher R, et al. Inguinal and ilio-inguinal lymphadenectomy in management of palpable melanoma lymph node metastasis: A long-term prospective evaluation of morbidity and quality of life. Ann Surg Oncol. 2019; 26(13):4663–4672.Article Pubmed
[9]Soydemir GP, Bahat Z, Kandaz M, Canyilmaz E, Yöney A. Prognostic factors and clinical course of extremity soft-tissue sarcomas. J Cancer Res Ther. 2020; 16(4):903–908.Article Pubmed
[10]Gervais MK, Hong NJL, McCready DR, Petrella T, Wright FC. Melanoma, multicenter selective lymphadenectomy Trial II [NCT00297895]; Surg Oncol Springer. 2016; 171–188.
[11]Polterauer S, Schwameis R, Grimm C, Hillemanns P, Jückstock J, et al. Lymph node ratio in inguinal lymphadenectomy for squamous cell vulvar cancer: Results from the AGO-CaRE-1 study. Gynecol Oncol. 2019; 153(2):286–229.Article Pubmed
[12]Torgbenu E, Luckett T, Buhagiar MA, Chang S, Phillips JL. Prevalence and incidence of cancer related lymphedema in low and middle-income countries: A systematic review and meta-analysis. BMC Cancer 2020; 20(1):604.Article Pubmed
[13]Mozzillo N, Caracò C, Marone U, Di Monta G, Crispo A, et al. Superficial and deep lymph node dissection for stage III cutaneous melanoma: clinical outcome and prognostic factors. World J Surg Oncol. 2013; 11:36.Article Pubmed
[14]Steffensen SM, Sørensen JA. Femoral hernia, a rare complication following deep inguinal lymph node dissection. BMJ Case Rep. 2015; 2015:bcr2014208177.Article Pubmed
[15]Desimone CP, Elder J, Nagell JRV. Selective inguinal lymphadenectomy in the treatment of invasive squamous cell carcinoma of the vulva. Int J Surg Onc. 2011; 2011:284374.Article Pubmed
[16]Boldo E, Mayol A, Lozoya R, Coret A, Escribano D, et al. Laparoscopically assisted ilio-inguinal lymph node dissection versus inguinal lymph node dissection in melanoma. Melanoma Manag. 2020; 7(2):MMT42.Article Pubmed
[17]Somé OR, Diallo M, Konkobo D, Yabré N, Konségré V, et al. Inguinal lymph node dissection for advanced stages of plantar melanoma in a low-income country. J Skin Cancer. 2020; 2020:8854460.Article Pubmed
[18]Sopracordevole F, Clemente N, Giorda G, Canzonieri V, Alessandrini L, et al. Number of nodes removed with inguinofemoral lymphadenectomy and risk of isolated groin recurrence in women with FIGO stage IB-II squamous cell vulvar cancer. Int J Gynecol Cancer. 2018; 28(8):1600–1605.Article Pubmed
[19]Watkin N. Is minimally invasive inguinal node dissection the way forward BJU Int. 2017; 119(4):505–506.Article Pubmed
[20]Brincat MR, Baron YM. Sentinel lymph node biopsy in the management of vulvar carcinoma: An evidence-based insight. Int J Gynecol Cancer. 2017; 27(8):1769–1773.Article Pubmed


