Journal of Neurology and Therapeutics
An International Peer-Reviewed Open Access Journal
ISSN 2397-1304


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Neurology and Therapeutics
Volume 3, Issue 1, September 2024, Pages 1–3
Case reportOpen Access
Optic perineuritis and orbital inflammation secondary to zoledronate: A case report
- 1 Department of Neurology, Baylor College of Medicine, Houston, TX 77030, United States
- 2 Department of Neurology, Michael E. DeBakey VA Medical Center, Houston, TX 77030, United States
*Corresponding author: César E. Escamilla-Ocañas, MD, Department of Neurology, Baylor College of Medicine, Houston, TX 77030, United States. Email: cescamilla-ocanas@mgh.harvard.edu
Received 23 May 2024 Revised 26 August 2024 Accepted 4 September 2024 Published 18 September 2024
DOI: http://dx.doi.org/10.14312/2397-1304.2024-1
Copyright: © 2024 Escamilla-Ocañas CE, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Introduction: Zoledronic acid is a potent bisphosphonate used to manage osteoporosis, Paget’s disease, hypercalcemia of malignancy, multiple myeloma, and metastatic bone disease. While generally well tolerated, bisphosphonates can have ocular side effects, including orbital inflammation and optic neuritis. Optic perineuritis (OPN), an inflammation of the optic nerve sheath, is an underreported zoledronate side effect. Case report: We describe a 75-year-old male with osteoporosis who developed left eye pain, ptosis, swelling, diplopia, and headache after a 4 mg zoledronate infusion. Examination showed left eye periorbital edema, erythema, ptosis, conjunctival injection, proptosis, and severe tenderness. Imaging revealed left orbital fat stranding and bilateral optic nerve sheath enhancement. Infectious and inflammatory work-ups were negative. Diagnosed with zoledronate-associated orbital inflammation, he was treated with high-dose intravenous methylprednisolone, resulting in significant improvement. Zoledronate was discontinued, and no symptoms recurred over a year. Ocular inflammation is a rare adverse effect of bisphosphonates, with neuro-ophthalmic complications being even rarer. Conclusion: This is the first reported case of OPN linked to zoledronate. Prompt recognition and treatment of this rare adverse effect are crucial to prevent permanent visual impairment.
Keywords: optic perineuritis; orbital inflammation; zoledronate; zoledronic acid
IntroductionTop
Zoledronic acid, or zoledronate, is a highly efficacious third-generation bisphosphonate, widely indicated for the prevention and treatment of osteoporosis, Paget’s disease of the bone, hypercalcemia of malignancy, multiple myeloma, and metastatic bone disease [1-3]. Its mechanism of action involves the inhibition of farnesyl diphosphate synthase, preventing osteoclastic bone loss and resorption [1]
Mostly bisphosphonates are well tolerated, and commonly reported side effects include fever, myalgias, influenza-like symptoms, headaches, arthralgias, atrial fibrillation, renal impairment, and osteonecrosis of the jaw [1]. More recently, ocular side effects such as, orbital inflammation, uveitis, scleritis, conjunctivitis and iritis have been reported during post-marketing use [4-7]. Additionally, more serious adverse effects, such as retrobulbar optic neuritis (ON) have also been previously reported [8-10]. Despite these reports ocular symptoms remain an underrecognized side-effect by the Food and Drug Administration (FDA) or pharmaceutical manufacturers [11].
Optic perineuritis (OPN) is an inflammatory process involving the optic nerve sheath. Its etiology and pathophysiology remain poorly understood and is often described as an idiopathic process [12]. In this paper, we present a case of orbital inflammation and bilateral OPN following a single infusion of zoledronate.
Case reportTop
A 75-year-old male with past medical history of osteoporosis, restless leg syndrome, tension headaches, drug induced parkinsonism, chronic kidney disease (CKD) presented to our hospital with 2 days history of left eye pain, ptosis, swelling, diplopia, and a left-sided retro-orbital headache starting one day after receiving an infusion of 4 mg zoledronic acid indicated for osteoporosis.
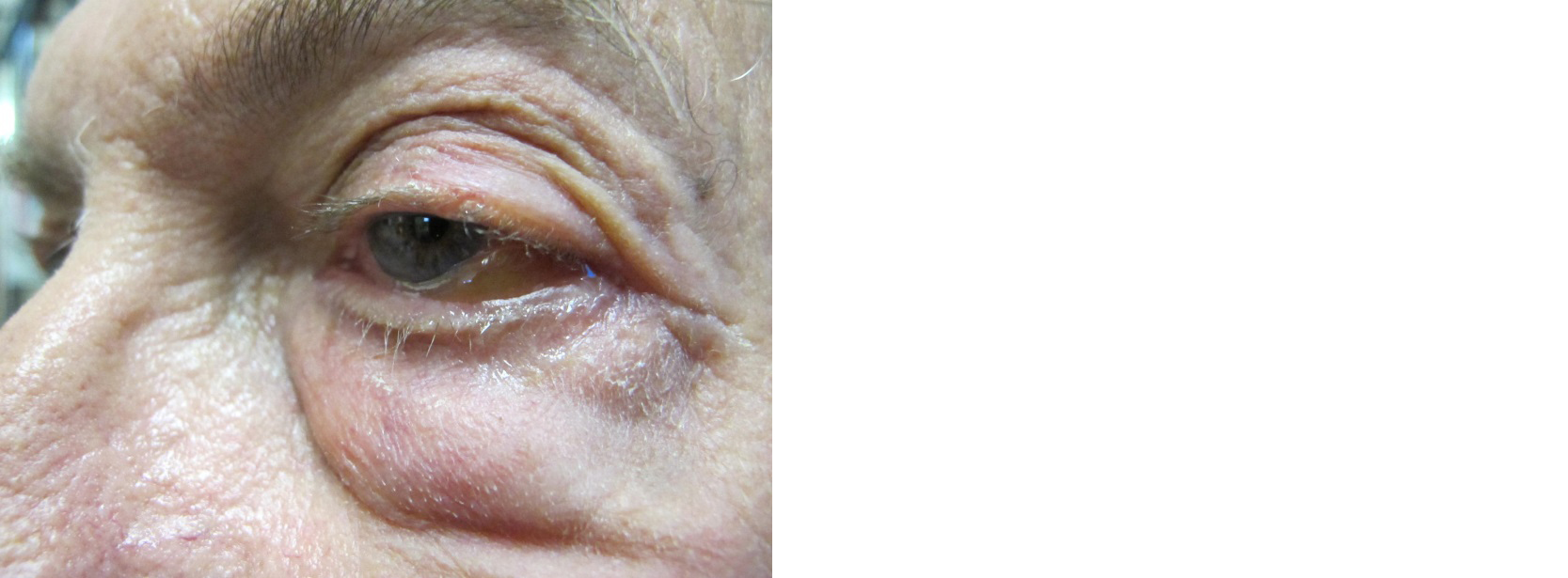
On exam, he was found to have left eye periorbital edema, erythema, ptosis, conjunctival injection, proptosis, severe periorbital tenderness (Figure 1), mildly impaired left eye abduction and adduction (extraocular movements were limited due to severe pain). Visual acuity was 20/20 bilaterally, pupils were equal and reactive to light, there was no relative afferent pupillary reflect and no evidence of optic nerve edema per fundoscopic examination.
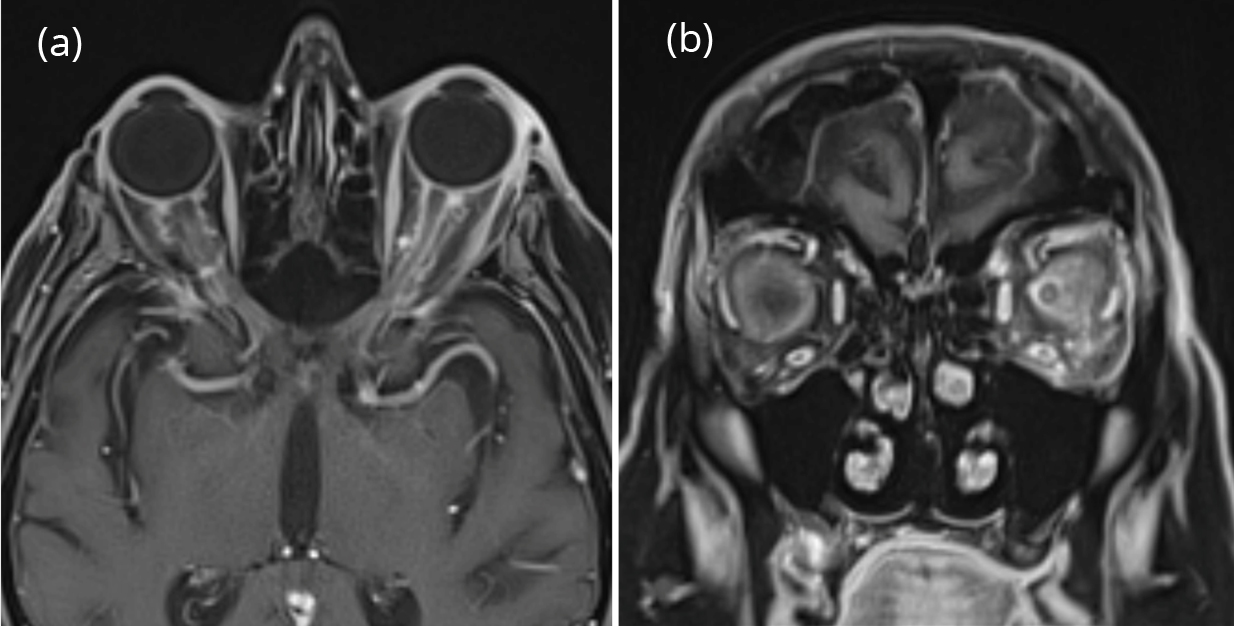
Computer tomography (CT) of the head and orbits showed left orbital fat stranding within the inferolateral aspect. Subsequently, magnetic resonance imaging (MRI) of the brain and orbit w/wo contrast showed bilateral optic nerve sheath enhancement, as well as left peri-orbital fat stranding (Figure 2). Vessel imaging showed no evidence of sinus thrombosis or other vascular abnormalities. A thorough infectious, rheumatologic, and inflammatory work-up were also negative (make a table of findings). Since his symptoms started right after receiving the zoledronate infusion and the work up for infectious and secondary inflammatory disorders was negative, he was treated as zoledronate associated orbital inflammation. High-dose intravenous methylprednisolone (IV MP) was started. Patient demonstrated significant improvement after 3 doses of IV MP and was discharged home on day 5 of hospitalization. His zoledronate infusions were discontinued following discharge and he has had no symptom recurrence after one year.
DiscussionTop
Ocular inflammation is a rare, but well-established adverse effect associated with administration of bisphosphonate. Uveitis, scleritis, conjunctivitis and orbital edema have been frequently reported. Ocular adverse effects associated specifically to zoledronate administration have been reported in less than 1% of patients [5-7]. Neuro-ophthalmic complications have also been reported but are even rarer. In the existing literature, we were only able to identify three cases of ON associated with zoledronate therapy [8-10]. Notably, all three cases had good outcomes and at least partial resolution of symptoms. Other bisphosphonates, such as pamidronate have also been previously associated to ON [13].
OPN also known as perioptic neuritis, is an uncommon form of orbital inflammatory disease characterized by inflammation of the optic nerve sheath. The etiology and pathological mechanisms of how inflammation develops and invades the nerve sheath in OPN remains poorly understood [12]. Generally, it has been classified as an idiopathic inflammatory condition or secondary to autoimmune, infiltrative, or infectious diseases including Grave’s disease, IgG-4 related disease, granulomatosis with polyangiitis (GPA), systemic lupus erythematosus (SLE), sarcoidosis, tuberculosis, toxoplasmosis, and cytomegalovirus [14]. All these secondary disorders were excluded in our case. OPN presents with pain, disc edema and progressive blurry vision, mimicking optic neuritis, but there is no optic nerve dysfunction, and the intracranial pressure is normal. MRI helps to differentiate between the two. Interestingly, no medication has been previously associated to OPN. To our knowledge, this is the first reported case of OPN attributed to zoledronate exposure.
Previously reported mechanisms associated with the bisphosphonate induced orbital inflammation include the activation of gamma delta T-cells, the initiation of an acute inflammatory response in extraocular muscles followed by the release of acute-phase reactants and cytokines such as interleukin-1, interleukin-6 and the activation of pro-inflammatory macrophages [5]. We theorize these identified mechanisms may extend into the meningeal membranes that surround the optic nerve, leading to OPN.
Although isolated orbital inflammation and other ocular adverse effects have been previously associated with zoledronate administration, neither ON nor OPN have been acknowledged by regulatory agencies.
ConclusionTop
OPN has never been reported as a potential side effect of zoledronate therapy. OPN is not typically self-limited and can lead to progressive visual deterioration without appropriate treatment. Moreover, ocular side effects previously associated to bisphosphonate use, tend to recur if the medication is resumed [15]. Therefore, this unusual but potentially serious side effect of zoledronate, should be recognized promptly to allow for appropriate treatment as well as discontinuation of the offending drug to avoid permanent visual impairment.
Conflicts of interest
Authors declare no conflict of interest.
ReferencesTop
[1]Reid IR, Green JR, Lyles KW, Reid DM, Trechsel U, et al. Zoledronate. Bone. 2020; 137:115390.Article Pubmed
[2]Qaseem A, Forciea MA, McLean RM, Denberg TD. Clinical Guidelines Committee of the American College of Physicians; Barry MJ, Cooke M, Fitterman N, Harris RP, Humphrey LL, Kansagara D, McLean RM, Mir TP, Schünemann HJ. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017; 166:818–839.Article Pubmed
[3]Kravets I. Paget's disease of bone: Diagnosis and treatment. Am J Med. 2018; 131:1298–1303.Article Pubmed
[4]Khalid MF, Daigle P, DeAngelis D, Micieli JA. Zoledronic acid-induced orbital inflammation. BMJ Case Rep. 2021; 14:e245359.Article Pubmed
[5]Phillips PM, Newman SA. Orbital inflammatory disease after intravenous infusion of zoledronate for treatment of metastatic renal cell carcinoma. Arch Ophthalmol. 2008; 126:137–139.Article Pubmed
[6]Schwab P, Harmon D, Bruno R, Fraunfelder FW, Kim DH. A 55-year-old woman with orbital inflammation. Arthritis Care Res (Hoboken). 2012; 64:1776–1782.Article Pubmed
[7]Keren S, Leibovitch I, Ben Cnaan R, Neudorfer M, Fogel O, et al. Aminobisphosphonate-associated orbital and ocular inflammatory disease. Acta Ophthalmol. 2019; 97:e792–e799.Article Pubmed
[8]Brulinski P, Nikapota AD. Zolendronic acid-induced retrobulbar optic neuritis: A case report. Clin Oncol (R Coll Radiol). 2013; 25:328–329.Article Pubmed
[9]Stack R, Tarr K. Drug-induced optic neuritis and uveitis secondary to bisphosphonates. N Z Med J. 2006; 119:U1888.Pubmed
[10]Lavado FM, Prieto MP, Osorio MRR, Gálvez MIL, Leal LM. Bilateral retrobulbar optic neuropathy as the only sign of zoledronic acid toxicity. J Clin Neurosci. 2017; 44:243–245.Article Pubmed
[11]Zometa. Prescribing information. Available from: Article
[12]Gupta S, Sethi P, Duvesh R, Sethi HS, Naik M, et al. Optic perineuritis. BMJ Open Ophthalmol. 2021; 6:e000745.Article Pubmed
[13]des Grottes JM, Schrooyen M, Dumon JC, Body JJ. Retrobulbar optic neuritis after pamidronate administration in a patient with a history of cutaneous porphyria. Clin Rheumatol. 1997; 16:93–95.Article Pubmed
[14]Li H, Zhou H, Sun J, Wang H, Wang Y, et al. Optic perineuritis and its association with autoimmune diseases. Front Neurol. 2021; 11:627077.Article Pubmed
[15]Chartrand NA, Lau CK, Parsons MT, Handlon JJ, Ronquillo YC, et al. Ocular side effects of bisphosphonates: A review of literature. J Ocul Pharmacol Ther. 2023; 39:3–16.Article Pubmed



