Journal of Modern Human Pathology
An International Peer-Reviewed Open Access Journal
ISSN 2397-6845

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Modern Human Pathology
Volume 2, Issue 1, January 2017, Pages 1–6
Original researchOpen Access
Comparison of the clinicopathologic features of solitary and multifocal papillary thyroid carcinoma and the utility of testing an additional tumor nodule for BRAFV600E in multifocal cases
- 1 Department of Pathology and Laboratory Medicine, Boston University School of Medicine, 670 Albany Street, Boston, MA 02118, USA
- 2 Lowell General Hospital, 295 Varnum Ave., Lowell, MA 01854, USA
*Corresponding author: Dr. Josenia Tan, Department of Pathology and Laboratory Medicine, Boston University School of Medicine, Boston Medical Center, 670 Albany Street, Boston, MA 02118, USA. E-mail: Josenia.Tan@bmc.org
Received 27 October 2016 Revised 16 December 2016 Accepted 23 December 2016 Published 29 December 2016
DOI: http://dx.doi.org/10.14312/2397-6845.2017-1
Copyright: © 2017 Tan J, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
A retrospective review of the tumor focality and BRAFV600E mutational status of all thyroidectomy cases diagnosed with papillary thyroid carcinoma (PTC) between 1/2010 and 7/2013 was performed. A total of 122 cases were included in this study consisting of 65 solitary cases and 57 multifocal cases with a total of 209 tumor nodules. In the multifocal tumors with absent BRAFV600E mutation in the dominant nodule, an additional tumor focus was submitted for testing. The clinicopathologic characteristics of the cases were then compared and analyzed. Significant association with BRAFV600E mutation was driven by previously established unfavorable histologic features such as usual variant, capsular invasion, lymphovascular invasion, extrathyroidal extension, and lymph node metastasis. We did not establish this association with tumor multifocality in this study. Although more cases of mPTC (multifocal papillary thyroid carcinoma) had BRAFV600E mutation in comparison with the solitary cases (56 vs 44%), we did not find a significant difference between the two groups (p = 0.0689). Furthermore, at least one of the unfavorable histologic features was also noted in three out of the five multifocal cases with discordant mutations in the two largest nodules. The heterogeneity of BRAFV600E mutational status in mPTC is evident in this study and although it seems prudent to further test additional foci, it appears that in most cases there are already histologic features that support a more aggressive behavior. Selective testing based on histomorphology may be a more practical approach in these cases.
Keywords: papillary thyroid carcinoma; microcarcinoma; solitary; multifocal; BRAFV600E; mutation; dominant nodule; heterogeneity
IntroductionTop
Papillary thyroid carcinoma (PTC) is the most common cancer of the thyroid accounting for more than 80% of all cases among all races. The incidence has been steadily increasing through the years with currently estimated new diagnosis of 60,220 and 1,850 deaths in the United States in 2013 [1]. PTC is often initially diagnosed with the use of fine needle aspiration (FNA) of thyroid nodules. It is also a common incidental finding in many patients undergoing thyroidectomy for non-malignant causes such as multinodular goiter and chronic thyroiditis [2, 3]. The steady increase in the number of cases has also been attributed to the incidental finding of papillary thyroid microcarcinomas (PTMC) in up to 79.3% of cases [3-6]. About 20% are categorized as multifocal PTC (mPTC) with a dominant nodule (> 1cm) and/or multiple microcarcinomas (≤ 1cm) [7-9].
Concurrent with these reports is the refinement of the approach to diagnosis and prognosis of PTC with the use of molecular techniques both at the FNA cytology and surgical histology levels. Several genetic alterations have been reported in PTC including RET, NTRK1, RAS and BRAF [10, 11]. BRAFV600E mutation is reported to be the most prevalent and specific genetic alteration in PTC and has been utilized for both diagnosis and prognosis. When this mutation is present, it has a strong diagnostic value for PTC when cytology yields an indeterminate result [12]. This mutation is particularly associated with more aggressive subtypes especially the tall cell variant. It is also reported to be present in anaplastic carcinoma especially those that arose from PTC. The presence of this mutation is associated with a more aggressive behavior including extrathyroidal extension, lymph node metastasis, advance stage, tumor recurrence and reduced sensitivity to radioiodine therapy [13, 14].
At our institution, testing for BRAFV600E mutation is also utilized post-surgically after a definite diagnosis of PTC for the management of patients. We noticed a few cases of mPTC’s with aggressive pathologic features such as capsular invasion, lymphovascular invasion, extrathyroidal extension and lymph node metastasis with absent BRAFV600E mutation in the tested dominant nodule. This study therefore aims to determine the rate of BRAFV600E mutation in cases of PTC with multifocal nodules when this mutation is absent in the dominant nodule. The clinicopathological characteristics of the cases are compared with the solitary tumors with and without mutation.
Materials and methodsTop
733 patients underwent thyroid lobectomy, subtotal to total thyroidectomy at Boston Medical Center from 1/2010 to 7/2013. 277 cases were diagnosed with PTC. BRAFV600E assay was performed on 128 cases, 122 of which were included in this study. Six cases were excluded due to insufficient diagnostic material for testing. Results of the testing were abstracted from the records and the corresponding clinicopathologic features recorded. This was done in accordance with the Boston Medical Center Internal Review Board (H-32597). The cases were segregated according to tumor focality and their BRAFV600E mutation status. The number and size of tumor nodules were also recorded. All multifocal tumors with absent mutation in the dominant nodule were reviewed and an additional tumor focus was submitted for BRAFV600E assay if not done previously. Features of aggressiveness including capsular and lymphovascular invasion, distant metastasis and number of regional lymph nodes were then recorded and compared. Fisher's exact test was used to examine the association between the different variables.
Tissue preparation
The tissue was fixed in 10% formalin and paraffin-embedded and thereafter sectioned. Sections were stained for hematoxylin & eosin and submitted for microscopic examination. The diagnosis of PTC was rendered based on the characteristic nuclear features of overlapping, ground-glass nuclei, nuclear pseudoinclusions and nuclear grooves. The cases were further classified according to the diagnosed histologic variant. For our study, cases were grouped into usual, follicular, microcarcinoma and other histologic variants. Tumor nodules ≤ 1 cm were classified as microcarcinoma.
BRAFV600E assay
The block containing the lesion was submitted for BRAFV600E assay. The H&E stained sections were reviewed by the submitting pathologist and the area of the lesion is identified. In specimens with multifocal tumors, the block with the largest nodule was marked and submitted for the assay. Multifocal tumors with absent BRAFV600E mutation in the dominant nodule were reviewed and the second largest tumor focus was submitted for BRAF assay. DNA was extracted by proteinase K digestion and boiling method. DNA was quantified with spectrophotometer and further diluted to different working concentrations for PCR assay. Allele-specific PCR (AS-PCR) was performed in duplicates with DNA concentrations ranging from 25 to 100 ng per PCR reaction. PCR products were run on a 3% agarose gel and mutation status was compared and assayed with a positive and negative control.
ResultsTop
A total of 122 cases of PTC were included in this study (Table1). The median age was 47 years, range 22-79 and included 97 females and 25 males with females predominating at a ratio of about 4:1. The cases consisted of 65 solitary tumors and 57 mPTC’s. The solitary and dominant tumor nodules were classified histologically at 29% (36) usual variant, 31% (37) follicular variant, 38% (47) microcarcinomas and two others consisting of Warthin-like and mixed pattern. The rate of BRAFV600E mutation among all cases was 47% (57). Capsular invasion was identified in 23% (28), lymphovascular invasion in 9% (11), extrathyroidal extension in 14% (17) and lymph node metastasis in 16% (19) cases.
| Number (%) | ||
| Age year, median (range) | 47 (22-79) | |
| Gender | ||
| Female | 97 (80%) | |
| Male | 25 (20%) | |
| Tumor focality | ||
| Solitary | 65 (53%) | |
| Multifocal | 57 (47%) | |
| PTC Variant | ||
| Usual | 36 (29%) | |
| Follicular | 37 (31%) | |
| Microcarcinoma | 47 (38%) | |
| Other | 2 (2%) | |
| BRAFV600E mutation status | ||
| Mutation present | 57 (47%) | |
| Mutation absent | 65 (53%) | |
| Capsular invasion | 28 (23%) | |
| Lymphovascular invasion | 11 (9%) | |
| Extrathyroidal extension | 17 (14%) | |
| Lymph node metastasis | 19 (16%) | |
The clinicopathological characteristics between the solitary and multifocal tumors are compared in Table 2. There was no age or gender difference between solitary vs multifocal tumors (p = 0.2762 and 0.1780 respectively). Furthermore, there were no significant differences between these two groups based on aggressive histological features such as capsular invasion, lymphovascular invasion, and lymph node metastasis, however extrathyroidal extension was noted to be higher in multifocal tumors (p = 0.0391).
| Solitary, 65 (53%) | Multifocal, 57 (47%) | p | ||
| Age year | ||||
| <45 | 29 (45%) | 32 (56%) | 0.2762 | |
| >45 | 36 (55%) | 25 (44%) | ||
| Gender | ||||
| Female | 55 (85%) | 42 (74%) | 0.178 | |
| Male | 10 (15%) | 15 (26%) | ||
| Capsular invasion | 13 (20%) | 15 (26%) | 0.5181 | |
| Lymphovascular invasion | 3 (5%) | 8 (14%) | 0.1112 | |
| Extrathyroidal extension | 5 (8%) | 12 (21%) | 0.0391 | |
| Lymph node metastasis | 7 (11%) | 12 (21%) | 0.1385 | |
| Tumor Nodules | Solitary, n = 65 | Multifocal, n = 144 | ||
| PTC Variant | ||||
| Usual | 14 (21%) | 27 (19%) | 0.7073 | |
| Follicular | 18 (28%) | 21 (14%) | 0.0341 | |
| Microcarcinoma | 31 (48%) | 96 (67%) | 0.014 | |
| Other | 2 (3%) | 0 (0%) | N/A | |
| Nodule size in cm | ||||
| ≤ 2 | 51 (78%) | 126 (88%) | 0.1008 | |
| 2.1 - 4 | 8 (12%) | 12 (8%) | 0.014 | |
| ≥ 4.1 | 6 (10%) | 6 (4%) | 0.0121 | |
A total of 209 nodules were identified from all cases consisting of 65 solitary nodules and 144 nodules from the cases with mPTC’s (Figure 1). They are compared further down in Table 2. The follicular histologic variant of PTC comprised a significantly higher proportion of the solitary cases (p = 0.0341). On the other hand, microcarcinomas were identified at a significantly higher rate in multifocal tumors (p = 0.0140). Of note was the usual variant which showed no difference in distribution between the two groups (p = 0.7073). When they were compared by tumor size driven by AJCC cancer staging (pT1: tumor size 2 cm or less; pT2: tumor more than 2 cm but not more than 4 cm and pT3: tumor more than 4 cm, all limited to the thyroid) [15], a difference was noted only with nodules larger than 2 cm (pT2, p = 0.0140) and more than 4 cm (pT3, p = 0.0121). Tumor nodules measuring 2 cm or larger were found more frequently in solitary PTC’s.
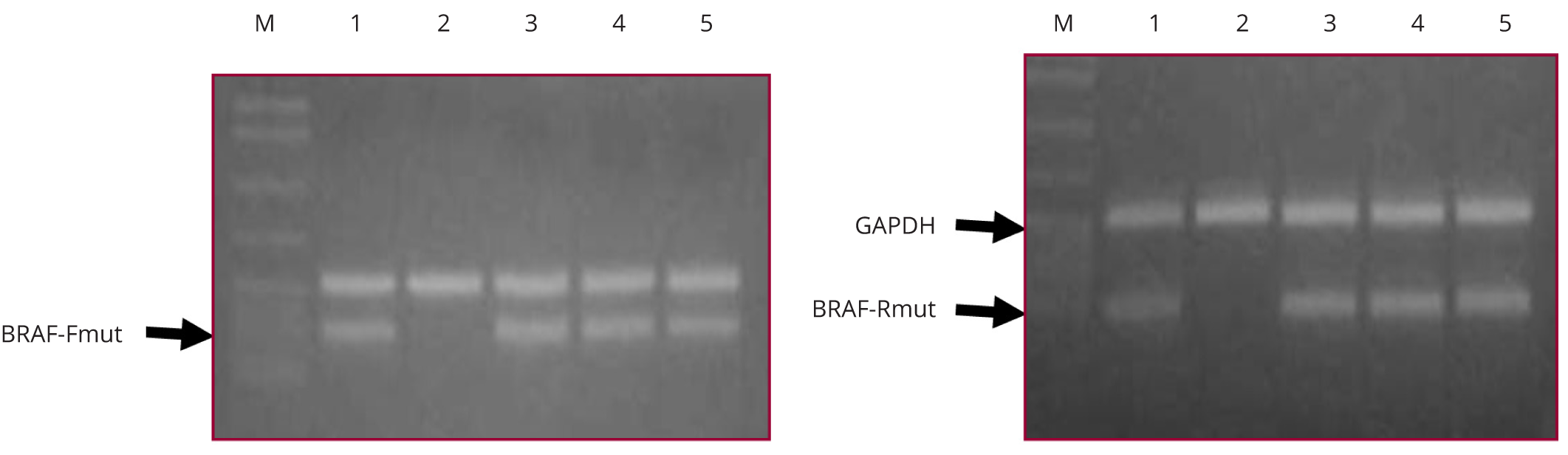
Table 3 compares the cases according to their BRAFV600E mutation status. There was no difference in the rate of BRAFV600E mutation between solitary and multifocal tumors (p = 0.0689). Capsular invasion, lymphovascular invasion, extrathyroidal extension and lymph node metastasis were significantly more frequent in cases with BRAFV600E mutation (p = 0.0045, 0.0234, 0.0029, 0.0004 respectively). There were no differences noted between the cases with 1, 2 or more than 3 tumor nodules. Among the 209 nodules identified from all cases consisting of 65 solitary nodules and 144 nodules from the cases with mPTC’s, a total of 152 tumor nodules were tested for BRAFV600E mutation. When the tumor nodules that were tested were compared according to histologic variant, there was a significant association noted between the usual variant and the presence of BRAFV600E mutation (p = 0.0001). An inverse relationship was noted with the follicular variant. There was no association noted when the tumor nodules were stratified according to size per pathologic T classification (pT1 vs pT2 vs pT3).
| Mutation present, n = 57 | Mutation absent, n = 65 | p | ||
| Unifocal (65) | 25 (38%) | 40 (62%) | 0.0689 | |
| Multifocal (57) | 32 (56%) | 25 (44%) | ||
| Capsular invasion | 20 (35%) | 8 (12%) | 0.0045 | |
| Lymphovascular invasion | 9 (16%) | 2 (3%) | 0.0234 | |
| Extrathyroidal extension | 14 (25%) | 3 (5%) | 0.0029 | |
| Lymph node metastasis | 16 (28%) | 3 (5%) | 0.0004 | |
| Number of nodules | ||||
| 1 | 25 (44%) | 40 (62%) | 0.0689 | |
| 2 | 18 (32%) | 17 (26%) | 0.5514 | |
| ≥ 3 | 14 (24%) | 8 (12%) | 0.0999 | |
| Tumor Nodules, n = 152 | n = 57 | n = 95 | ||
| PTC variant | ||||
| usual | 27 (47%) | 11 (12%) | 0.0001 | |
| follicular | 5 (9%) | 35 (37%) | 0.0001 | |
| micro carcinoma | 24 (42%) | 48 (50%) | 0.4017 | |
| other | 1 (2%) | 1 (1%) | N/A | |
| Nodule size (cm) | ||||
| ≤ 2 | 49 (86%) | 70 (74%) | 0.1035 | |
| 2.1-4 | 5 (9%) | 15 (16%) | 0.3216 | |
| ≥ 4.1 | 3 (5%) | 10 (10%) | 0.3726 | |
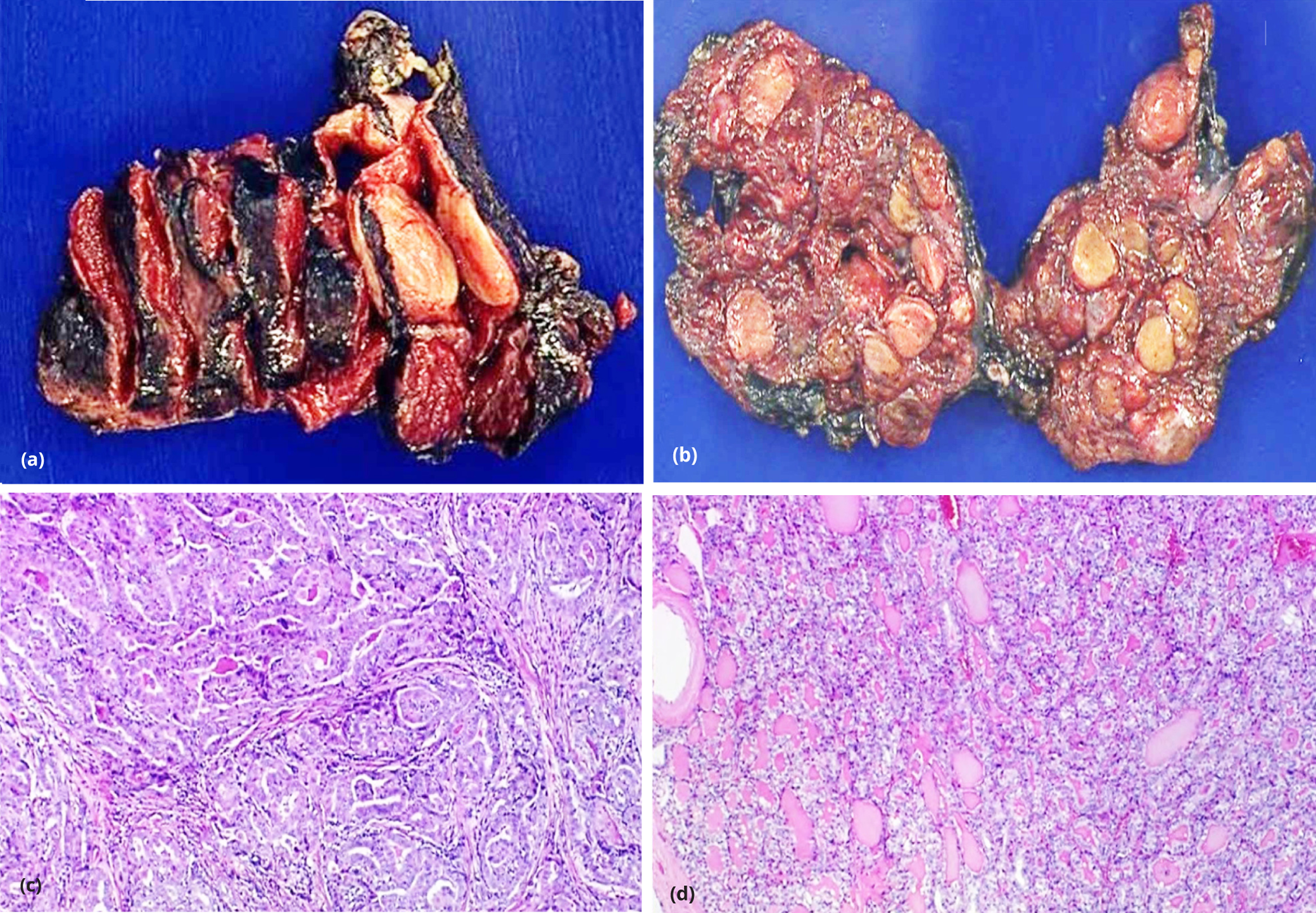
Five out of the 32 multifocal cases were found to have a heterogenous BRAFV600E mutational status after testing the second largest tumor nodule in the cases that showed no mutation in the dominant nodule. Table 4 shows a tabulation of the histopathologic features of these cases. The number of tumor foci ranged from 2 to 5, with four cases arising from the same lobe. The nodule size tested for BRAFV600E mutation ranged from 0.5 to 3 cm. Three of the five nodules with heterogenous BRAFV600E mutation already displayed at least 2 of the following aggressive histopathologic features: capsular invasion, lymphovascular invasion, extrathyroidal extension and lymph node metastasis. Two cases did not show any of these histopathologic features but the second nodule tested positive for BRAFV600E mutation (Figure 2).
| Case | #1 | #2 | #3 | #4 | #5 | |
| Number of tumor foci | 5 | 4 | 2 | 3 | 2 | |
| Location | Bilateral | Unilateral | Unilateral | Unilateral | Unilateral | |
| *Histologic variant | Usual | Usual | Follicular | Usual | Micro | |
| Size of the tumor nodule tested (cm) | ||||||
| Dominant nodule (BRAFV600E wild type) | 3 | 2.3 | 1.4 | 1.5 | 0.9 | |
| Non-dominant nodule (BRAFV600E mutant) | 1.4 | 0.8 | 0.5 | 1.2 | 0.5 | |
| Histopathology | ||||||
| Capsular invasion | (+) | (-) | (-) | (+) | (-) | |
| Lymphovascular invasion | (-) | (-) | (-) | (+) | (-) | |
| Extrathyroidal extension | (-) | (+) | (-) | (+) | (-) | |
| Lymph node metastasis | (+) | (+) | (-) | (+) | (-) | |
Note: *Histologic variant of nodules > 1 cm, otherwise classified as microcarcinoma.
DiscussionTop
BRAFV600E is the most common genetic mutation in PTC and many studies have found association with adverse prognostic features including more aggressive subtypes, extrathyroidal extension, lymph node and distant metastasis, as well as increased recurrence and mortality [16, 17]. Melck and colleagues also demonstrated that cases of PTC with BRAFV600E mutation were more likely to require cervical reoperation compared to those without mutation [18]. The overall rate of BRAF mutation in PTC has been reported up to 83% [19, 20]. The presence of this mutation has also been reported to have a higher incidence in mPTC [21]. Given a significant number of cases has this mutation and the association with adverse features, the possibility of missing the presence of this mutation when testing only the dominant nodule in mPTC’s may affect the overall prognosis for these patients.
Most studies report a rate of mPTC’s in the range of 20-27% but has been reported to be present in up to 78.1% of patients, usually with a dominant tumor nodule and multiple microcarcinomas [7, 8, 16, 22]. The number of tumor nodules usually ranges from two to six with a mean size of about 1 cm [16, 24]. This study reports a rate of 47% for multifocal cases. And consistent with other reports, majority of the mPTC cases consisted of multiple microcarcinomas and were classified as pT1. The only histologic feature we found to be significantly associated with multifocality was extrathyroidal extension (p = 0.0391), however the other features such as capsular invasion, lymphovascular invasion, and lymph node metastasis appeared to be more frequent in mPTC. The increased proportion of follicular variant PTC in the solitary cases in our study may explain why these aggressive features were less frequent in this group. These findings allude to previous reports correlating multifocality with a more aggressive behavior [7, 9, 22, 25]. Various endeavors to elucidate the origin and behavior of mPTC include both independent clonal origin of these tumors as well as intraglandular spread and subsequent clonal evolution to acquire a more aggressive phenotype [4, 11, 24, 26]. Demonstration of the heterogeneity of BRAFV600E mutation in mPTC’s also further supports the independent clonal origin of these tumors [18, 26]. These varying propositions on the origin of these tumors can affect our practice of tumor nodule selection for ancillary studies which include BRAFV600E mutation. Would testing the dominant tumor nodule be sufficient in cases of mPTC?
In this study, we report a rate of 56% for BRAFV600E mutation in mPTC. This rate included five cases (9%) with discordant mutational status when the second largest nodule was tested. We note that our rate is lower than reported in other studies which found discordance in up to 40% of the cases [26, 27]. Although more cases of mPTC had BRAFV600E mutation in comparison with the solitary cases (56 vs 44%), we did not find a significant difference between the two groups (p = 0.0689). We also did not find a significant association with the number or size of the nodules. Our findings however, are concordant with other studies in showing the landmark histologic features associated with BRAFV600E mutation such as the usual histologic variant, capsular invasion, lymphovascular invasion, extrathyroidal extension, and lymph node metastasis. Furthermore, at least one of these features was also noted in three out of the five multifocal cases with discordant mutations in the two largest nodules. The cases which did not show any of these histologic features were subtyped as follicular variant and microcarcinomas. On further review of the microcarcinomas, they were determined to demonstrate follicular subtypes.
PTC generally has a good prognosis with a 10-year survival rate of over 90% after appropriate treatment; however, recurrence rate is reported in up to 32% of cases [1, 28]. Disease progression and recurrence have been associated with unfavorable pathologic features such as extrathyroidal extension, lymph node and distant metastasis, advanced pathologic stage (Stage III/IV), as well as the presence of BRAFV600E mutation [29, 30]. There are also reports associating multifocality with a more aggressive behavior [9, 25].
Limitations
Our limitations include those characteristic of a retrospective study. We also did not test all the nodules in the mPTC cohort which probably explains our lower rate compared to other studies. Except for those with discordant mutational status, the microcarcinomas were not further subtyped. Given there are reports associating multifocality with adverse prognosis [9, 25], we recommend continued efforts towards elucidating the adverse features specific to multifocal cases, at both the histologic and molecular levels. Of note, all the cases of follicular variant papillary thyroid carcinoma were classified under the old nomenclature as reported in the original diagnosis. Although, subclassification of encapsulated and non-encapsulated variants was not done, the low incidence of BRAFV600E mutation in this group appears reflective of the new classification and nomenclature.
ConclusionTop
We showed in this study that the mutational status in the dominant nodule in mPTC’s does not necessarily reflect the mutational status of all the tumors in the thyroid including microcarcinomas. It is probably prudent to test the second dominant tumor nodule in mPTC’s for BRAFV600E mutation; however the decision must be done in conjunction with other histologic features which are already likely to be present and are predictive of a more aggressive behavior. Selective testing may be a more practical approach in these cases.
Conflicts of interest
The authors declare no conflicts of interest.
ReferencesTop
[1]Seer stat fact sheets: Thyroid cancer.Website
[2]Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras NS, et al. The association between thyroid carcinoma and Hashimoto's thyroiditis: The ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010; 20(8):873–878.Article Pubmed
[3]Pelizzo MR, Rubello D, Bernardi C, Gemo G, Bertazza L, et al. Thyroid surgical practices shaping thyroid cancer incidence in North-Eastern Italy. Biomed Pharmacother. 2014; 68(1):39–43.Article Pubmed
[4]Vasileiadis I, Karakostas E, Charitoudis G, Stavrianaki A, Kapetanakis S, et al. Papillary thyroid microcarcinoma: Clinicopathological characteristics and implications for treatment in 276 patients. Eur J Clin Invest. 2012; 42(6):657–664.Article Pubmed
[5]Nucera C, Pontecorvi A. Clinical outcome, role of BRAFV600E, and molecular pathways in papillary thyroid microcarcinoma: is it an indolent cancer or an early stage of papillary thyroid cancer Front Endocrinol (Lausanne). 2012; 3:33.Article Pubmed
[6]Londero SC, Krogdahl A, Bastholt L, Overgaard J, Trolle W, et al. Papillary thyroid microcarcinoma in Denmark 1996-2008: A national study of epidemiology and clinical significance. Thyroid. 2013; 23(9):1159–1164.Article Pubmed
[7]So YK, Kim MW, Son YI. Multifocality and bilaterality of papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol. 2015; 8(2):174–178.Article Pubmed
[8]Kuo SF, Lin SF, Chao TC, Hsueh C, Lin KJ, et al. Prognosis of multifocal papillary thyroid carcinoma. Int J Endocrinol. 2013; 2013:809382.Article Pubmed
[9]Lin JD, Chao TC, Hsueh C, Kuo SF. High recurrent rate of multicentric papillary thyroid carcinoma. Ann Surg Oncol. 2009; 16(9):2609–2616.Article Pubmed
[10]Nikiforov YE. Molecular analysis of thyroid tumors. Mod Pathol. 2011; 24 Suppl 2:S34–43.Article Pubmed
[11]Bansal M, Gandhi M, Ferris RL, Nikiforova MN, Yip L, et al. Molecular and histopathologic characteristics of multifocal papillary thyroid carcinoma. Am J Surg Pathol. 2013; 37(10):1586–1591.Article Pubmed
[12]Ohori NP, Nikiforova MN, Schoedel KE, LeBeau SO, Hodak SP, et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of "follicular lesion of undetermined significance/atypia of undetermined significance". Cancer Cytopathol. 2010; 118(1):17–23.Article Pubmed
[13]Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: A meta-analysis. J Clin Endocrinol Metab. 2012; 97(12):4559–4570.Article Pubmed
[14]Prescott JD, Sadow PM, Hodin RA, Le LP, Gaz RD, et al. BRAFV600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery. 2012; 152(6):984–990.Article Pubmed
[15]Edge SB, Byrd DR. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17(6):1471–1474.Article Pubmed
[16]Zheng X, Xia T, Lin L, Gao S, Lee Y, et al. BRAFV600E status and clinical characteristics in solitary and multiple papillary thyroid carcinoma: Experience of 512 cases at a clinical center in China. World J Surg Oncol. 2012; 10:104.Article Pubmed
[17]Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246(3):466–470.Article Pubmed
[18]Melck AL, Yip L, Carty SE. The utility of BRAF testing in the management of papillary thyroid cancer. Oncologist. 2010; 15(12):1285–1293.Article Pubmed
[19]Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005; 12(2):245–262.Article Pubmed
[20]Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004; 45(5):818–821.Article Pubmed
[21]Zheng XQ, Wang C, Xu M, Yu Y, Yun XW, et al. Progression of solitary and multifocal papillary thyroid carcinoma – a retrospective study of 368 patients. Chin Med J (Engl). 2012; 125(24):4434–4439.Article Pubmed
[22]Lin YK, Sheng JM, Zhao WH, Wang WB, Yu XF, et al. Multifocal papillary thyroid carcinoma: clinical analysis of 168 cases. Zhonghua Wai Ke Za Zhi. 2009; 47(6):450–453.Pubmed
[23]Katoh R, Sasaki J, Kurihara H, Suzuki K, Iida Y, et al. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma. A clinicopathologic study of 105 consecutive patients. Cancer. 1992; 70(6):1585–1590.Article Pubmed
[24]Jovanovic L, Delahunt B, McIver B, Eberhardt NL, Grebe SK. Most multifocal papillary thyroid carcinomas acquire genetic and morphotype diversity through subclonal evolution following the intra-glandular spread of the initial neoplastic clone. J Pathol. 2008; 215(2):145–154.Article Pubmed
[25]Qu N, Zhang L, Ji QH, Zhu YX, Wang ZY, et al. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer. 2014; 14:914.Article Pubmed
[26]Giannini R, Ugolini C, Lupi C, Proietti A, Elisei R, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007; 92(9):3511–3516.Article Pubmed
[27]Park SY, Park YJ, Lee YJ, Lee HS, Choi SH, et al. Analysis of differential BRAFV600E mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer. 2006; 107(8):1831–1838.Article Pubmed
[28]Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22(11):1144–1152.Article Pubmed
[29]Ito Y, Miyauchi A. Prognostic factors of papillary and follicular carcinomas in Japan based on data of kuma hospital. J Thyroid Res. 2012; 2012:973497.Article Pubmed
[30]Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, et al. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008; 93(10):3943–3949.Article Pubmed



