Journal of Modern Human Pathology
An International Peer-Reviewed Open Access Journal
ISSN 2397-6845

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Modern Human Pathology
Volume 2, Issue 2, November 2017, Pages 7–12
Original researchOpen Access
A comprehensive review of nephroblastoma with ureteric involvement
- 1 Department of Anatomical Pathology, National Health Laboratory Service & School of Laboratory Medicine & Medical Sciences, University of KwaZulu-Natal, Durban, KwaZulu-Natal, South Africa
- 2 Department of Paediatric Surgery, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Inkosi Albert Luthuli Central Hospital, Durban, KwaZulu-Natal, South Africa
*Corresponding author: PK Ramdial, Department of Anatomical Pathology, Level 3, Laboratory Building, Inkosi Albert Luthuli Central Hospital, 800 VusiMzimela Road, Mayville, 4058, KwaZulu-Natal, South Africa. Tel.: +27 (0)31 2402693; Facsimile: +27(0)31 2402610; E-mail: ramdialpk@gmail.com; ramdial@ukzn.ac.za
Received 29 July 2017 Revised 26 October 2017 Accepted 10 November 2017 Published 24 November 2017
DOI: http://dx.doi.org/10.14312/2397-6845.2017-2
Copyright: © 2017 Singh S, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Ureteric involvement is described rarely in nephroblastoma, the most common pediatric renal tumor. This clinicopathological, descriptive retrospective study was conducted to elucidate the prevalence and histomorphological features of ureteric involvement by nephroblastoma. Of 454 nephroblastomas diagnosed in the 25-year study period, 32 displayed ureteric involvement; 21 and 11 demonstrated prolapse and invasion, respectively. The patient cohort had a mean age of 47.3 months and mainly advanced stage disease. Pre-operative radiological and intra-operative assessments identified ureteric involvement in 4 and 13 patients, respectively, but distinction between ureteric prolapse and invasion was not possible. Histopathological assessment of the primary renal tumor demonstrated exclusive triphasichistomorphology in all 32 nephroblastomas. Favorable histology, diffuse anaplasia and nephroblastomatosis were present in 28, 4 and 7 tumors, respectively. Re-appraisal of 17 post-treated tumors were classified by SIOP criteria as mixed(6), stromal(4), anaplastic(4) and regressive(3) types. The ureteric component displayed triphasic(11), biphasic(5) and monophasic(1)histomorphology. The staging profile of patients with ureteric prolapse was stages I(3), II(5), III(6), IV(6) and V(1). The staging profile of patients with ureteric invasion was stages I(0), II(2), III(3), IV(4) and V(2). Distant metastases were present in 10/32 patients. Follow-up of 32 patients confirmed 21 that were tumor-free, 7 with recurrent disease and 4 fatalities; of those that remained tumor-free, 11 had advanced disease. Even in advanced tumor stages, complete excision of the urinary tract tumor and optimal treatment of disseminated malignancy are pivotal to overall patient management and outcome.
Keywords: nephroblastoma; Wilms’ tumor; ureter; invasion; prolapse; kidney; renal
IntroductionTop
Nephroblastoma (Wilms’ tumor) is the most common pediatric renal neoplasm, accounting for approximately 85% of reported childhood renal tumors [1]. They are characterized by a restricted pattern of local growth and metastasis [2]. The former involves the perirenal soft tissues, adrenal glands, bowel, liver, vertebrae and paraspinal region [2]. While involvement of the renal collecting system is common [3], extension into the ureter is reported uncommonly, mainly as case reports [4-8]. In their landmark study of 42 children with nephroblastoma and ureteric involvement, Ritchey et al [9] highlighted the absence of direct tumor invasion through the ureteric wall. Two other case reports presented similar findings [10, 11]. Despite nephroblastomas being the commonest childhood renal tumor in KwaZulu-Natal [12], ureteric involvement has not been formally evaluated in this center, to date.
This study was therefore undertaken to determine the prevalence and nature of ureteric involvement by nephroblastoma and the histopathological spectrum, stage and outcome thereof.
Materials and methodsTop
This is a descriptive retrospective study that involved a re-appraisal of all patients with nephroblastomas with ureteric involvement from the databases of the Departments of Paediatric Surgery and Anatomical Pathology, Inkosi Albert Luthuli Central Hospital, Durban, South Africa, from the beginning of 1988 to the end of 2012. The clinicopathological details that were accessed from the departmental archives included patient age, sex, race, presenting symptoms and outcome and tumoral imaging findings, recurrence, metastasis, stage and morphological subtype as defined by the International Society of Paediatric Oncology (SIOP) criteria [13], presence of nephroblastomatosis and pattern of ureteric involvement. The SIOP histological subtype was not performed in cases for which only archival reports were available because of insufficient information. The outcome was assessed as per follow-up records. The most recent follow-up chart entry represented the last follow-up visit. Patients that did not receive pre-operative chemotherapy were excluded from the study. The patterns of ureteric involvement included prolapse of nephroblastoma into the ureteric lumen and ureteric wall invasion. The former was recognized by the presence of urothelium-lined pelvicalyceal tumor within the ureteric lumen with a “pushing” growth pattern in the absence of invasion of ureteric mural micro-anatomic components. Ureteric wall invasion was characterized by variable ureteric replacement by tumor, including tumoral invasion of the serosa, muscularispropria, subepithelial stroma and urothelium in transverse and/or longitudinal directions. The study was approved by the Bio-Ethics Research Committee of the University of KwaZulu-Natal (Study number: BE005/13).
ResultsTop
Of a total of 454 nephroblastomas that were diagnosed in the 25-year study period, 35 demonstrated ureteric involvement. Of these, 32(7%) patients received pre-operative chemotherapy and form the study cohort. While only reports were available for 15/32 tumors, reports, slides and blocks from 17/32 tumors were available for review. The records of the latter 17 patients were accessed for the radiographic, gross and microscopic images contained in Figures 1, 2 and 3.
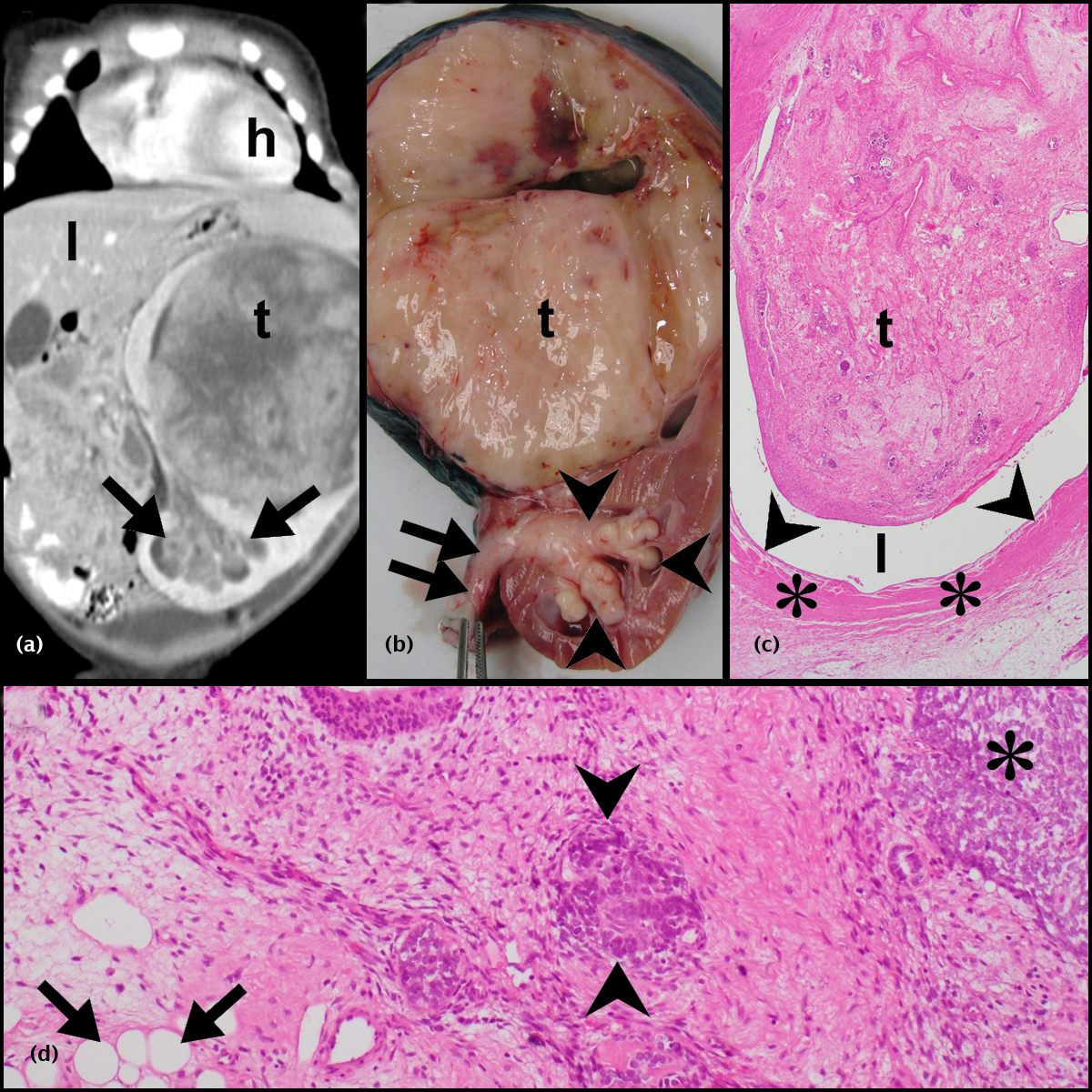
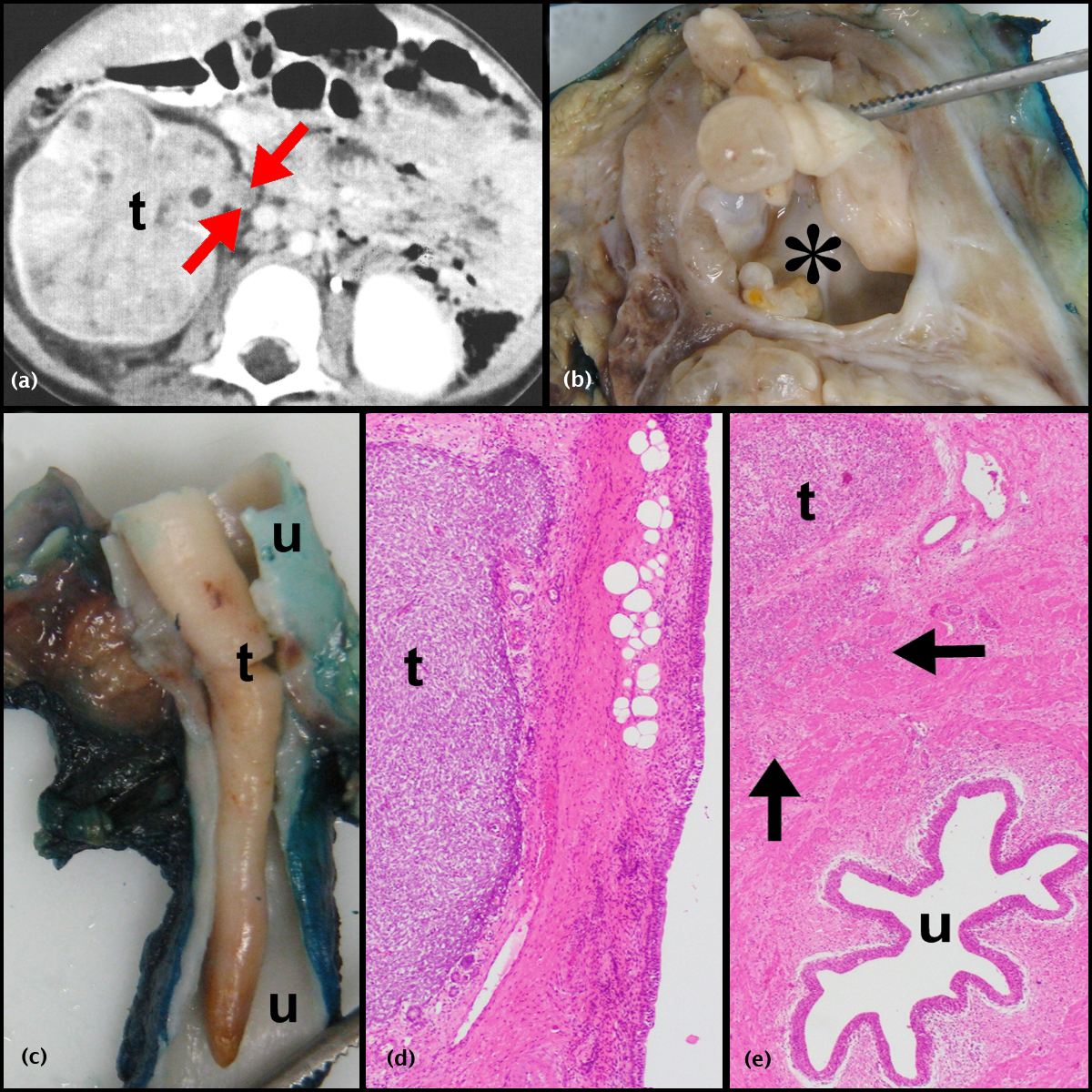
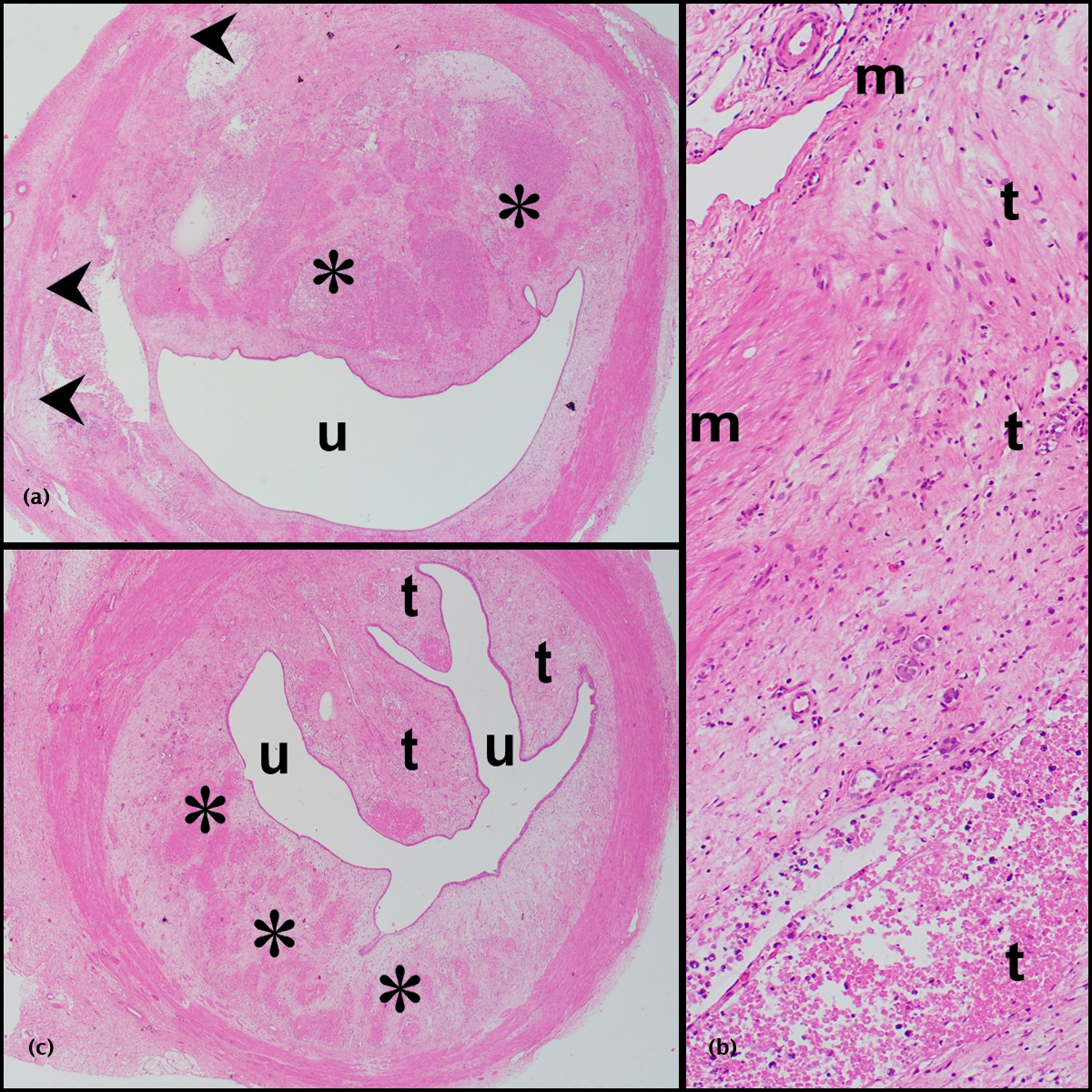
Clinical features
Thirty of 32 (93.8%) patients presented with an abdominal mass; 9/32 (28.1%) also had hematuria (Table 1). Four of 32 pre-operative CT scans demonstrated ureteric involvement but it was not possible to differentiate between prolapsing and invasive ureteric disease radiologically (Figures 1a and 2a). Surgical tumor excision was undertaken in 31/32 patients; 1/32 died prior to surgery. Ureteric involvement was identified intra-operatively in 13/31 (41.9%) patients. As per surgical protocol, ureteric excision was performed as low as possible; where ureteric involvement was recognized, excision was undertaken as low as possible distal to the involved segment. Thirty of 31 patients received post-operative chemotherapy (patients 4, 5, 7, 8 and 11), radiotherapy (patient 14) or a combination of both treatment modalities (patients 1-3, 6, 10, 12, 13, 15-20, 22-32). Patient 9 did not receive post-operative chemotherapy due to leukopenia and anemia. The patient remained tumor-free but was lost to follow-up 2 months post tumor excision.
| No | Age/Sex | Hematuria | Invasion/rupture | SIOP | Ureteric involvement by tumor | Outcome | ||
| Stage | Histological subtype | Nature | Morphology | |||||
| 1 | 3/Male | Present | Confined to kidney | I | Not done | Prolapse | *Not described | Tumor-free: 154 months |
| 2 | 2/Male | Present | Confined to kidney | I | Not done | Prolapse | *Not described | Tumor-free: 129 months |
| 3 | 4/Female | Present | Confined to kidney | I | Anaplastic | Prolapse | Triphasic | Tumor-free: 52 months |
| 4 | 2/Female | Absent | Lymphatic/renal vein | II | Not done | Prolapse | *Not described | Tumor-free: 6 months |
| 5 | 3/Male | Absent | Renal sinus/perinephric fat | II | Not done | Prolapse | *Not described | Tumor-free: 13 months |
| 6 | 3/Female | Absent | Through capsule | II | Not done | Prolapse | *Not described | Tumor-free: 66 months |
| 7 | 4/Female | Absent | Through capsule | II | Not done | Prolapse | *Not described | Tumor-free: 136 months |
| 8 | 2/Male | Absent | Through capsule | II | Not done | Prolapse | *Not described | Tumor-free: 41 months |
| 9 | 2/Male | Absent | Through capsule | II | Not done | Invasion | *Not described | Tumor-free: 2 months |
| 10 | 6/Female | Present | Perinephric fat | II | Not done | Invasion | *Not described | Tumor-free: 8 months |
| 11 | 3/Male | Present | Lymphatic/inferior vena cava/right atrium | III | Not done | Prolapse | *Not described | Recurrence: 24 months treated Tumor-free: 12 months |
| 12 | 3/Male | Present | Lymphatic/nodal | III | Not done | Prolapse | *Not described | Tumor-free: 11 months |
| 13 | 2.5/Female | Absent | Lymphatic | III | Not done | Prolapse | *Not described | Recurrence: 7 months treated Tumor-free: 8 months |
| 14 | 7/Female | Present | Lymphatic/inferior vena cava | III | Not done | Prolapse | *Not described | Tumor-free: 18 months |
| 15 | 2/Male | Absent | Lymphatic/nodal | III | Mixed | Prolapse | Biphasic | Tumor-free: 6 months |
| 16 | 3/Male | Absent | Rupture | III | Stromal | Prolapse | Monophasic | Tumor-free: 37 months |
| 17 | 6.5/Female | Absent | Rupture | III | Anaplastic | Invasion | Biphasic | Tumor-free: 12 months |
| 18 | 3.5/Male | Absent | Lymphatic/renal vein/ inferior vena cava/ nodal | III | Anaplastic | Invasion | Triphasic | Tumor-free: 25 months |
| 19 | 3/Male | Absent | Lymphatic/renal vein | III | Mixed | Invasion | Triphasic | Tumor-free: 52 months |
| 20 | 4/Female | Absent | Lymphatic/renal vein/nodal/ colon/ovary | IV | Not done | Prolapse | *Not described | Recurrence: 4 months treated Died at time of recurrence |
| 21 | 2/Male | Present | Renal vein /lung | IV | Mixed | Prolapse | Triphasic | Died: pre-operatively |
| 22 | 2.5/Male | Absent | Lymphatic/nodal/lung/rupture | IV | Mixed | Prolapse | Triphasic | Died: 57 months |
| 23 | 5.5/Male | Absent | Lymphatic/renal vein/ perinephric fat/spleen/liver | IV | Anaplastic | Prolapse | Triphasic | Died: 4 months |
| 24 | 1.5/Female | Absent | Renal vein /lung | IV | Mixed | Prolapse | Triphasic | Recurrence: 7 months treated Tumor-free: 9 months |
| 25 | 1/Male | Absent | Renal vein | IV | Regressive | Prolapse | Biphasic | Recurrence: 10months treated. |
| 2nd recurrence: 15 months later palliation | ||||||||
| 26 | 9/Male | Absent | Lymphatic/renal vein/ lung | IV | Not done | Invasion | *Not described | Recurrence: 8 months treated Died: 9 months |
| 27 | 10.5/Male | Present | Lymphatic /renal vein/liver/lung/ bladder/rupture | IV | Stromal | Invasion | Biphasic | Died: 13 months |
| 28 | 8/Male | Absent | Lymphatic/ nodal /liver | IV | Regressive | Invasion | Triphasic | Tumor-free: 51 months |
| 29 | 10/Female | Absent | Liver/diaphragm | IV | Regressive | Invasion | Triphasic | Tumor-free: 33 months |
| 30 | 4/Male | Absent | Lymphatic/renal vein | VsII | Stromal | Prolapse | Triphasic | Tumor-free: 32 months |
| 31 | 2.5/Female | Absent | Lymphatic/renal vein/nodal/spinal cord | VsIV | Mixed | Invasion | Triphasic | Tumor-free:36 months |
| 32 | 1/Male | Absent | Lymphatic/renal vein/ lung | VsIII | Stromal | Invasion | Biphasic | Recurrence: 4 months treated Tumor-free: 27 months |
Abbreviations: Age: in years; No: number; SIOP: International Society of Paediatric Oncology; *Not described: morphological details of ureteric component not described in reports: slides/blocks not available for review.
Pathological features
Of the 32 nephroblastomas, 21 (65.6%) demonstrated ureteric prolapse and 11(34.4%), invasive ureteric disease (Table 1). All 32 nephroblastomas had triphasichistomorphology (Figure 1d).
Nephroblastomas with ureteric prolapse (Figures 1a-d)
Of the 21 nephroblastomas, 12 had archival reports exclusively and 9 had reports, blocks and slides for study purposes. Based on diagnostic SIOP microscopic criteria [13], the nine tumors with available blocks and slides corresponded to mixed (4), stromal (2), anaplastic (2) and regressive (1) types, while the ureteric component had triphasic (6), biphasic (2) and monophasic (1) histomorphology. Lymphatic invasion was identified in 10. Nephroblastomatosis was present in 6 tumors, three were perilobar and three intralobar in type. While diffuse anaplasia was present in sections of the main renal tumor in two patients, it was not identified in the ureteric component of any study sample.
Nephroblastomas with ureteric invasion (Figures 2a-e and 3a-c)
Of 11 nephroblastomas, 3 had archival reports exclusively and 8 had reports, blocks and slides for study purposes. Based on diagnostic SIOP microscopic criteria [13], the latter eight corresponded to mixed (2), stromal (2), anaplastic (2) and regressive (2) types, while the ureteric component had triphasic (5) and biphasic (3) histomorphology. Lymphatic invasion was identified in seven and perilobarnephroblastomatosis in one nephroblastoma. While diffuse anaplasia was present in sections of the main renal tumor in two patients, it was not identified in the ureteric component of any study sample.
SIOP staging and outcome
Nephroblastomas with ureteric prolapse, invasion and incompletely excised ureteric tumor are currently staged as stage I, II or III tumors, respectively [13]. The ureteric component of all tumors was completely excised with confirmed tumor-free ureteric excision margins. All SIOP stages were encountered in the present study (Table 1). The staging profile of patients with ureteric prolapse was stages I(3), II(5), III(6), IV(6) and V(1). The staging profile of patients with ureteric invasion was stages I(0), II(2), III(3), IV(4) and V(2). Because of the extent of tumor invasion, rupture and spread, 18/21 (85.7%) nephroblastomas with ureteric prolapse and 9/11(81.8%) nephroblastomas with ureteric invasion presented with tumor beyond stage I and stage II, respectively (Table 1). Ten of 32(31.3%) patients had distant metastases to the lung, liver or spinal cord (Table 1); six and four of these patients had ureteric invasion and prolapse, respectively. The staging profile of the 21 tumor-free patients was stage I(3), II(7), III(7), IV(2) and V(2). None of the patients with tumor recurrences or who died had low stage I or II disease; 9/11 patients with recurrent disease or who died had stage IV and V disease (Table 1).
Twenty-one of the 32 patients remained tumor-free for the documented follow-up period which ranged from 2 to 154 months. Of the remaining 11 patients, seven developed recurrent disease within 4 to 24 months and four died (Table 1). Of the seven patients with tumor recurrence, four achieved remission post-treatment and 3 died of tumor. Of the four patients that died without tumor recurrence, three (patients 22, 23, 27) demised from non-tumor related causes and one (patient 21) died pre-operatively; autopsy findings of the latter patient confirmed death from disseminated, including pulmonary, nephroblastoma.
DiscussionTop
Nephroblastomas, first described by John Hunter in the 18th century [1], is the most common renal neoplasm in childhood, but is reported rarely in extra-renal locations and in adults [1, 14]. Derived from nephrogenic blastema, the histomorphologic features recapitulate varying stages of embryonic development [15]. Although the peak age incidence of nephroblastomas is two to three years, 98% occur in children below ten years [16]. The pluripotency of nephroblastoma is exalted by its variable, but pathognomonic, epithelial, stromal and blastemal features [17]. Chemosensitivity and radiosensitivity characteristics have rendered it a highly curable malignancy [18]. With cure rates in excess of 90% in developed countries, nephroblastoma is viewed as one of the therapeutic successes of modern medicine [16]. However, the survival rates are much lower in developing countries, with some sub-Saharan countries reporting 40% survival rates up to eight months after diagnosis [19]. Tumoralbilaterality, poor renal care and transplantation supportive resources, second primary pathology, late presentation and surgical challenges are possible causes for the intercontinental outcome-related differences [20, 21]. In common with the global experience, nephroblastoma is also the most common renal tumor of childhood in KwaZulu-Natal, South Africa [12]. Whilst several workers have expanded the clinicopathological profile of nephroblastoma from this geographic region [20, 22, 23], to date, ureteric involvement in patients with nephroblastoma has neither been reported in the provincial KwaZulu-Natal setting, nor from any national South African context.
The incidence of ureteric involvement of 7% in the present study is distinctly higher than the 2.5% described incidence in the NWTS-5 [9]. This may be due to the differing demographics of the study groups. Ritchey et al. [9] documented a median patient presentation age of 33 months. The mean patient presentation age in the present study was 47.3 months. While Ritchey and colleagues [9] described the SIOP staging profile in their study as stage I(10), II(18), III(14) and IV(3), the patients in the current study were staged as I(3), II(7), III(9), IV(10) and V(3). Advanced disease presentations in African patients for a variety of reasons are documented, including poor socioeconomic background and limited access to healthcare [20, 21, 24]. It is therefore tempting to speculate that the relatively higher prevalence of ureteric involvement in our study is a consequence of advanced disease stage and older patient age at presentation. Hematuria, a rare finding in classical nephroblastoma, is a relatively commonly described clinical feature in patients with ureteric involvement [4-9]. Identified in 9/32 (28.1%) of the current study patients, hematuria may be a pre-operative clinical marker for potential ureteric involvement. The largest single study [9] on ureteric involvement by nephroblastomas recorded ureteric extension in 14/42 (33.3%) patients on pre-operative imaging, in contrast to 4/32 (12.5%) patients in the present study. It remains conjectural whether the low frequency of radiographically-confirmed ureteric involvement is a function of the decreased disease exposure, rarity of the entity or lack of tumoral soft tissue dilatation of the ureter [9, 25].
The majority of reported cases in the global literature have demonstrated ureteric prolapse rather than invasion of the ureter wall [4-9, 13]. “Botryoid” nephroblastomas, postulated to arise from intralobar nephrogenic rests are also associated with extension into the renal collecting system and protrusion into ureter and bladder [2]. While Ritchey et al.[9] noted the presence of intralobar nephrogenic rests in 50% of their study cases, the same was noted in 3/7 (42.9%) patients with nephroblastomatosis in the present study. Although favorable histology has been recorded in the majority, anaplasia has been documented in < 10% of nephroblastomas with ureteric involvement [4-9]. The 5% incidence of anaplasia in classical nephroblastoma increases to 13% at five years of age [26]. In the present study, 28/32 (87.5%) nephroblastomas had favourable histology and 4/32 (12.5%) demonstrated diffuse anaplasia. Although hypothetical, this may be a reflection of the higher mean patient age of our study, as tumoral anaplasia increases with increasing patient age [9, 26, 27]. The histopathology of the ureteric component has not received much attention in the literature. The main renal component of all nephroblastomas in the current study were triphasic. The ureteric component was triphasic (64.7%), biphasic (29.4%) or monophasic (5.9%) and all contained a stromal component.
The prognostic implications of ureteric involvement depend on the nature of involvement and completeness of resection of the renal tumor viz. prolapse, invasion and incomplete excision of the ureteric component corresponding to stages I, II and III disease, respectively [13]. The outcome of these nephroblastomas is impacted predominantly by the presence of tumoral anaplasia, metastases and adequacy of resection [9, 25]. In the present study, 11/32 patients had recurrent disease or died; all 11 patients had higher than stage II disease, and 9 had stage IV and V disease. Whilst the disease outcome in these patients was therefore a reflection of disease stage rather than ureteric involvement per se, it is also pertinent that 11/21 patients with a tumor-free outcome over 6 to 52 months of follow-up, had stage III, IV and V disease. Although the disease stage is a function of the biological aggressiveness of the underlying renal tumor and host factors [26], it is arguable that ureteric involvement, specifically ureteric invasion, maybe a consequence of similar prognostic tumor and host characteristics. It is also debatable, especially in resource-constrained settings, such as ours, whether there is clinical and pathological value in investigating and optimally appraising the ureter for nephroblastoma involvement and adequacy of excision in patients with advanced tumor stage. That 11/21patients (Table 1) with higher stage disease remained tumor-free argues in favor of and rationalizes the role of full clinical and histopathological appraisal and staging of tumors. Furthermore, the aim of current nephroblastoma management schedules, within and beyond the confines of the kidney and ureter, is to control disease and improve patient quality of life. Chemotherapy, radiation and metastatectomy may be employed to ensure such management objectives [28], but the latter is relatively new practice in our center; metastatic disease in the study patients was managed by chemoradiation exclusively.
ConclusionTop
While ureteric involvement by nephroblastoma is a rarely documented phenomenon, it was identified in 7% of nephroblastomas over a 25-year-period in KwaZulu-Natal, South Africa. Even in advanced tumor stages, complete excision of the urinary tract tumor and optimal treatment of disseminated malignancy are pivotal to overall patient management and outcome. Increased global reporting of ureteric involvement by nephroblastoma is necessary to determine the exact significance of the different involvement patterns, especially when metastatic disease is well controlled with current and emerging therapeutic modalities [28]. More detailed prospective studies, including phenotypic, immunophenotypic and genotypic appraisal of the ureteric component of nephroblastomas that have not been exposed to neoadjuvant chemotherapy may be helpful in determining potential associations between ureteric involvement and the biological profile of the primary renal and contiguous ureteric tumoral components.
Acknowledgements
We thank Mr. KievershenNargan and Mr. Dinesh Sookhdeo for laboratory assistance and Mrs. M Moodley for administrative assistance.
Conflicts of interest
Authors declare no conflicts of interest.
ReferencesTop
[1]Vujanić GM, Sandstedt B. The pathology of Wilms’ tumour (nephroblastoma): The International Society of Paediatric Oncology approach. J ClinPathol. 2010; 63(2):102–109.Article Pubmed
[2]Ordóñez NG, Rosai J. Urinary tract. Kidney, renal pelvis and ureter: Bladder. In: Rosai and Ackerman’s Surgical Pathology, Tenth edition. Elsevier. 2011; 1101–1286.Article
[3]Lowe LH, Banks WJ, Allen TD. Urethral metastasis in Wilms’ tumor. J Urol. 1998; 160(1):165.Article Pubmed
[4]Breslow N, Churchill G, Beckwith JB, Fernbach DJ, Otherson HB, et al. Prognosis for Wilms’ tumor patients with nonmetastatic disease at diagnosis – results of the second National Wilms’ Tumor Study. J ClinOncol. 1985; 3(4):521–531.Article Pubmed
[5]Stevens PS, Eckstein HB. Ureteral metastasis from Wilms’ tumor. J Urol. 1976; 115(4):467–468.Article Pubmed
[6]Takyurt A. Wilms’ tumor at the lower end of the ureter extending to the bladder: Case report. J Urol. 1972; 107(1):142–143.Article Pubmed
[7]Watkins JP. Wilms’ tumor with ureteral metastases extending into the bladder. J Urol. 1957; 77(4):593–596.Article Pubmed
[8]Woodhead DM, Gigax JH, Wahle WH, Holcomb TM. Urothelial implantation of Wilms’ tumors. Ann Surg. 1968; 167(1):127–131.Article Pubmed
[9]Ritchey M, Daley S, Shamberger RC, Ehrlich P, Hamilton T, et al. Ureteral extension in Wilms’ tumor: A report from the National Wilms’ Tumor Study Group (NWTSG). J Pediatr Surg. 2008; 43(9):1625–1629.Article Pubmed
[10]Mitchell CS, Yeo TA. Noninvasive botryoid extension of Wilms’ tumor into the bladder. PediatrRadiol. 1997; 27(10):818–820.Article Pubmed
[11]Stanley K, Khoudary KP, Nasrallah PF. Urothelial extension of Wilms’ tumor presenting as a prolapsing urethral mass. J Urol. 1995; 153(6):1981–1983.Article Pubmed
[12]Ramdial PK, Hadley GP, Sing Y. Spinal cord compression in children with Wilms’ tumour. PediatrSurg Int. 2010; 26(4):349–353.Article Pubmed
[13]De Kraker J, Graf N, Pritchard-Jones K, Pein F. Nephroblastoma clinical trial and study SIOP 2001, Protocol. SIOP RTSG. 2001.
[14]Al-Hussain T, Ali A, Akhtar M. Wilms’ tumor: An update. AdvAnatPathol. 2014; 21(3):166–173.Article Pubmed
[15]Treetipsatit J, Raveesunthornkiet M, Ruangtrakool R, Sanpaki K, Thorner PS. Teratoid Wilms’ tumor: Case report of a rare variant that can mimic aggressive biology during chemotherapy. J Pediatr Surg. 2011; 46(12):e1–6.Article Pubmed
[16]Davidoff AM. Wilms’ tumor. CurrOpinPediatr. 2009; 21(3):357–364.Article Pubmed
[17]Myers JB, Dall’Ella J, Odom LF, McGavran L, Lovell MA, et al. Teratoid Wilms’ tumor, an important variant of nephroblastoma. J Pediatr Urol. 2007; 3(4):282–286.Article Pubmed
[18]Sultan I, Ajlouni F, Al-Jumaily U, Al-Ashhab M, Hashem H, et al. Distinct features of teratoid Wilms’ tumor. J Pediatr Surg. 2010; 45(10):e13–19.
[19]Stones DK, Hadley GP, Wainwright RD, Stefan DC. The impact of ethnicity on Wilms tumor: Characteristics and outcome of a South African cohort. Int J Pediatr. 2015; 2015:706058.Article Pubmed
[20]Hadley GP, Mars M, Ramdial PK. Bilateral Wilms’ tumour in a developing country: A descriptive study. PediatrSurg Int. 2013; 29(5):419–423.Article Pubmed
[21]Hadley LG, Rouma BS, Saad-Eldin Y. Challenge of pediatric oncology in Africa. SeminPediatr Surg. 2012; 21(2):136–141.Article Pubmed
[22]Govender D, Hadley GP, Nadhvi SS, Donnellan RB. Primary lumbosacral Wilms’ tumour associated with occult spinal dysraphism. Virchows Arch. 2000; 436(5):502–505.Article Pubmed
[23]Ramburan A, Hadley GP, Govender D. Expression of E-cadherin, cadherin-11, alpha-, beta- and gamma-catenins in nephroblastomas: Relationship with clinicopathological parameters, prognostic factors and outcome. Pathology. 2006; 38(1):39–44.Article Pubmed
[24]Visser YT, Uys R, van Zyl A, Stefan DC. Nephroblastoma - a 25-year review of a South African unit. J Med Life. 2014; 7(3):445–449.Pubmed
[25]Soccorso G, Sehuraman C, Al-Adnani M, Yeomanson D, Walker J. Ureteric extension of Wilms' tumour: A case report. J Pediatr Urol. 2013; 9(1):e3–5.Article Pubmed
[26]Murphy WM, Grignon DJ, Perlman EJ. Kidney tumours in children. In: Tumours of the kidney, bladder and related urinary structures, Fourth series. Edited by Murphy WM, Grignon DJ, Perlman EJ. Washington, American registry of pathology, 2004, p. 1–100.
[27]Zhou M, Magi-Galluzi C. Genitourinary pathology 2nd Edition. A volume in the series: Foundations in Diagnostic Pathology. Elsevier. 2007; 281–340.Article
[28]Berger M, Fernandez-Pineda I, Cabello R, Ramirez-Villar GL, Márquez-Vega C, et al. The relationship between the site of metastases and outcome in children with stage IV Wilms’ tumor: Data from 3 European pediatric cancer institutions. J PediatrHematolOncol. 2013; 35(7):518–524.Article Pubmed



