Journal of Modern Human Pathology
An International Peer-Reviewed Open Access Journal
ISSN 2397-6845

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Modern Human Pathology
Volume 3, Issue 7, December 2018, Pages 22–26
Case reportOpen Access
Inflammatory myofibroblastic tumor of the uterus that might STUMP you: Report of a case with clinicopathological and immunohistochemical findings
- 1 Department of Pathology, St. Luke’s Hospital System of Kansas City, Kansas City, Missouri, USA
- 2 Department of Obstetrics and Gynecology, St. Luke’s Hospital System of Kansas City, Kansas City, Missouri, USA
*Corresponding author: Ossama Tawfik, MD., PhD., Professor, Chairman of Pathology, MAWD Pathology Group, Saint Luke’s Health System, 4401 Wornall Road, Kansas City, MO 64111, USA. Tel: (816) 932-2447; Fax: (816) 935-8118; E-mail: otawfik@mawdpathology.com
Received 07 October 2018 Revised 23 November 2018 Accepted 27 November 2018 Published 01 December 2018
DOI: http://dx.doi.org/10.14312/2397-6845.2018-7
Copyright: © 2018 Tawfik O, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Inflammatory myofibroblastic tumor is an under-recognized locally aggressive tumor that has rarely been described in the uterus. We present a case in a 40-year-old patient with a large tumor mass in the posterior wall of her uterus. The patient was followed for years clinically and assumed to have a leiomyomatous lesion. The clinical, histopathological and immunohistochemical features are discussed with review of the literature.
Keywords: inflammatory myofibroblastic tumor; histopathology; uterus
IntroductionTop
Inflammatory myofibroblastic tumor (IMT) of the female genital tract is rare neoplasm with less than 75 cases reported in the literature [1-4]. The distinction between IMTs and other mesenchymal uterine tumors, especially those with myxoid changes can be subtle. Most of IMTs are indolent tumors with intermediate malignant potential and they don’t require aggressive approaches, radical surgeries or systemic chemotherapy as needed for other similar but aggressive mesenchymal uterine tumors such as leiomyosarcoma [2-4]. We present another case of this rare disease that was followed for years clinically and assumed to be a leiomyomatous lesion. The importance of careful histopathological and immunohistochemical evaluation and review of the literature for accurate diagnosis and management of these patients are discussed.
Case reportTop
The patient is 40-year-old gravida 5, para 5 female who presented with a four-year history of abnormal uterine bleeding (AUB) with symptomatic anemia requiring hospitalization and blood transfusions on several occasions, cervical dysplasia, dyspareunia, fatigue and lower abdominal pain. She reported debilitating, cramping lower abdominal pain, with heavy periods every two and a half to three weeks lasting for eight days and passing blood clots that flooded her clothes. The clinical impression was uterine fibroids with a slight concern for adenomyosis that was treated with oral contraceptive pills (OCPs), multiple Depo-Provera injections in addition to blood transfusion. A pap smear performed two years prior to her admission was reported as high risk human papillomavirus (HPV) positive atypical squamous cells with undetermined significance. A colposcopy was performed three weeks prior to her last admission revealing areas suspicious for high grade squamous intraepithelial lesion on endocervical curettage. The patient’s past medical history was significant for atrial fibrillation, hypothyroidism following thyroidectomy for Grave’s disease, depression, schizophrenia, osteoarthritis and irritable bowel syndrome. She attained menarche at age 12 years and used OCPs to control bleeding but had to stop due to undesirable weight gain. Her family history was noncontributory.
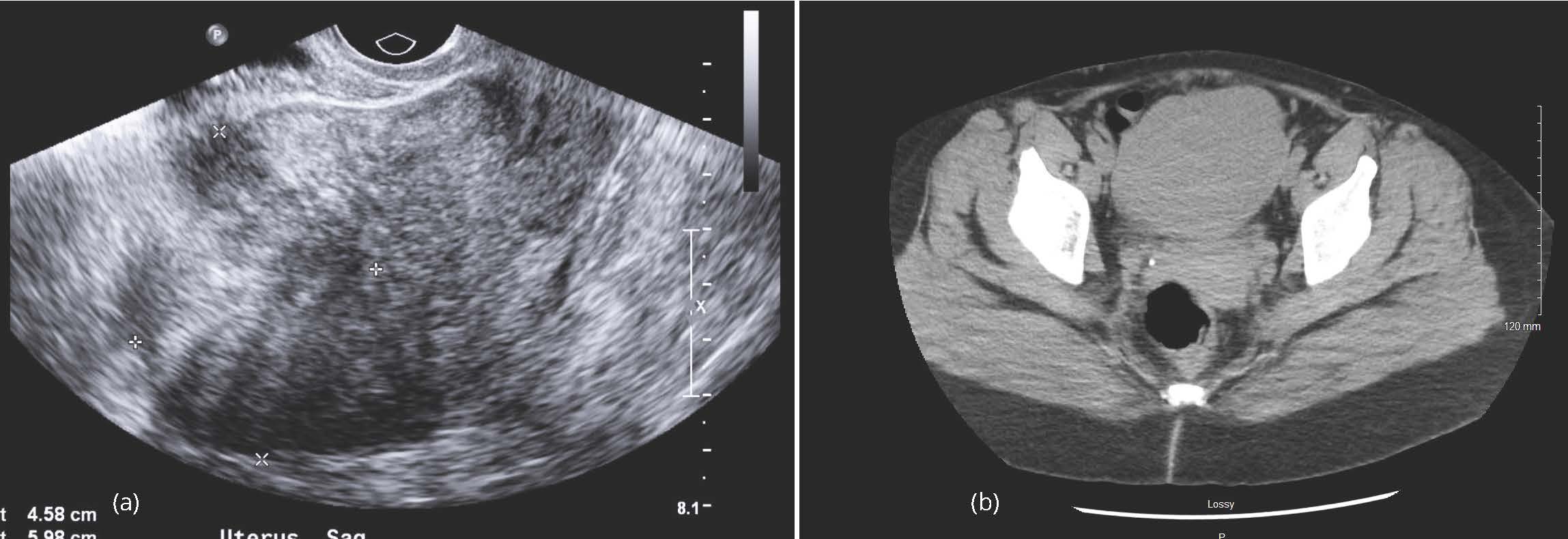
Physical examination revealed a mobile, globular uterus, roughly 10 weeks in size, with minimal descent. Tenderness was noted on deep palpation of the uterus. A transvaginal ultrasound of the pelvis revealed an enlarged lobular uterus measuring 10.7 × 7.5 × 7.4 cm (Figure 1a). Multiple uterine fibroids versus adenomyosis were noted with the largest lesion measuring 4.2 cm in the posterior uterine segment. Endometrial thickness was within normal limits. A 1.6 cm right ovarian hemorrhagic cyst was also noted. Computerized tomography (CT) scan of the abdomen and pelvis revealed similar findings with a mildly enlarged uterus with lobular contour (Figure 1b).
Because of her AUB and cervical dysplasia a hysterectomy was recommended. The patient underwent total laparoscopic hysterectomy with bilateral salpingectomy. Intraoperatively, the uterus was noted to be enlarged with deviation to the left and significant fullness in the posterior cul-de-sac, revealing a pedunculated, degenerating fundal mass with several adhesions to the bowel. The pedunculated mass drained viscous, yellow-brown, clear contents. Unremarkable ovaries and tubes were noted bilaterally. Upon removing the specimen, through the vagina the pedunculated mass got detached from the uterus. It was sent to pathology for intraoperative consultation. A diagnosis of spindle cell lesion was made on frozen section. The patient required one unit of packed red blood cells (PRBCs), but tolerated the procedure well. She was taken to the recovery room in a stable condition. Postoperatively the patient was noted to be anemic, requiring additional three units of PRBCs. The patient was discharged for follow up in two weeks with our gynecologic oncologist.
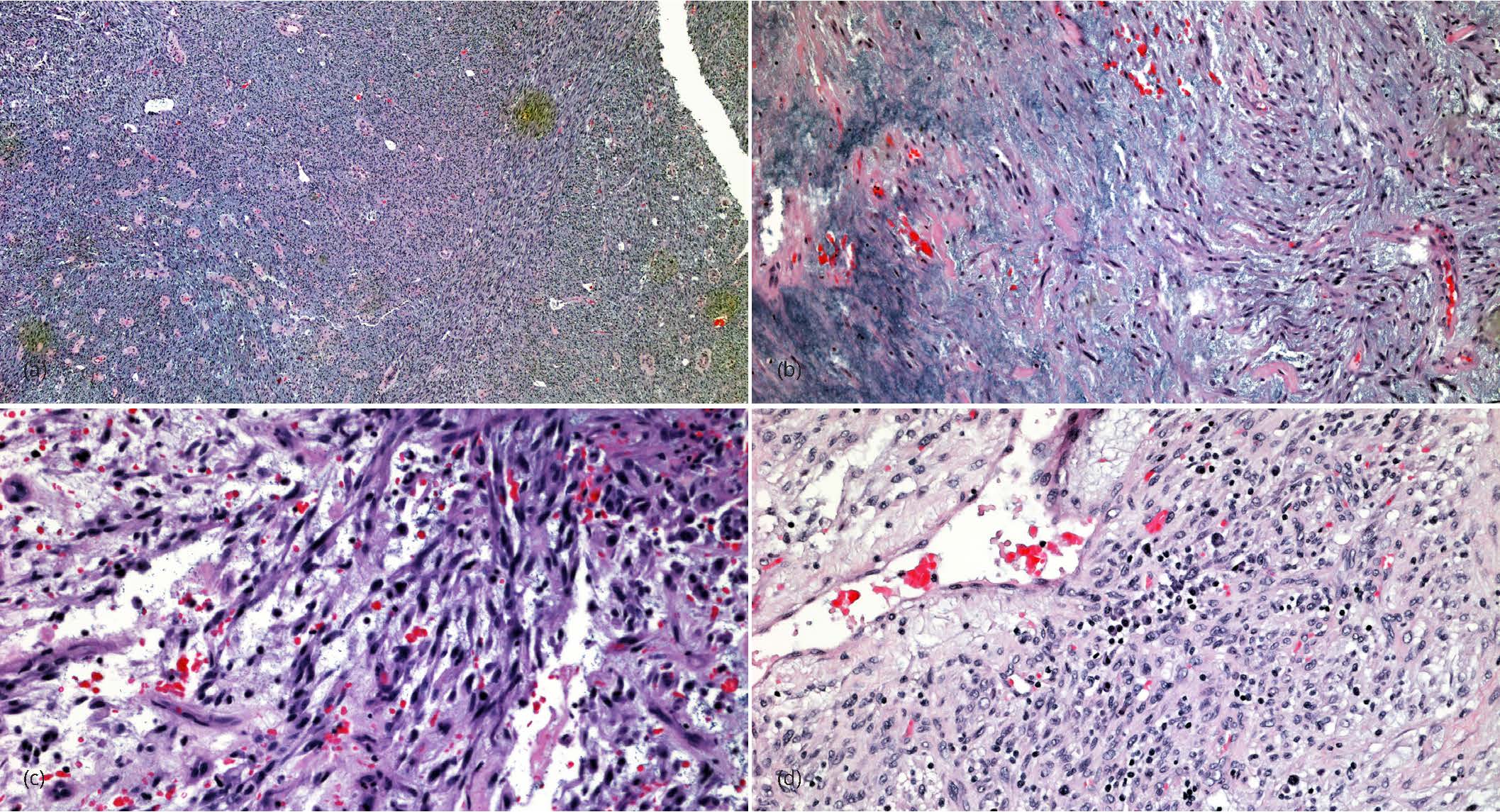
The surgical specimen consisted of the uterine pedunculated mass that was separated from the uterus during surgery and the hysterectomy specimen. The mass measured 9.0 × 6.5 × 2.5 cm. It was lobulated with a multicystic cut surface varying from 0.2 to 1.0 cm. It contained clear mucinous material. There were no papillary excrescences or other abnormalities noted. The uterus was enlarged weighing 280 gms. In addition to a tan lobulated area extending from the right cornu of the uterus similar to the pedunculated mass, the myometrium contained several small leiomyomas. The serosal surface was congested with numerous adhesions on the fundus posteriorly. The endometrium was unremarkable averaging 0.3 cm in thickness. The cervix and fallopian tubes were grossly unremarkable. On microscopic examination the mass was subserosal in location, well circumscribed and had adhesions to the surrounding organs. It is composed with bland spindle cells with a fascicular or storiform architecture. The tumor has a prominent myxoid background associated with a prominent vasculature, edematous changes and a striking lymphoplasmacytic infiltrate (Figure 2). Nuclear atypia was mild to moderate with inconspicuous nucleoli. There was no increase in mitotic activity averaging 2-3/10 high power fields and no lymphovascular invasion. A panel of immunostains showed the tumor cells to be strongly positive for anaplastic lymphoma kinase (ALK), CD10, desmin, focally positive for epithelial membrane antigen and negative for smooth muscle actin and h-caldesmon confirming the diagnosis of inflammatory myofibroblastic tumor (Figures 3). Ki67 confirmed the low proliferative activity of the tumor and ROS1 was also negative. Additional findings included a small residual focus of high grade squamous intraepithelial lesion, that was completely excised in the cervix and multiple small leiomyomata.
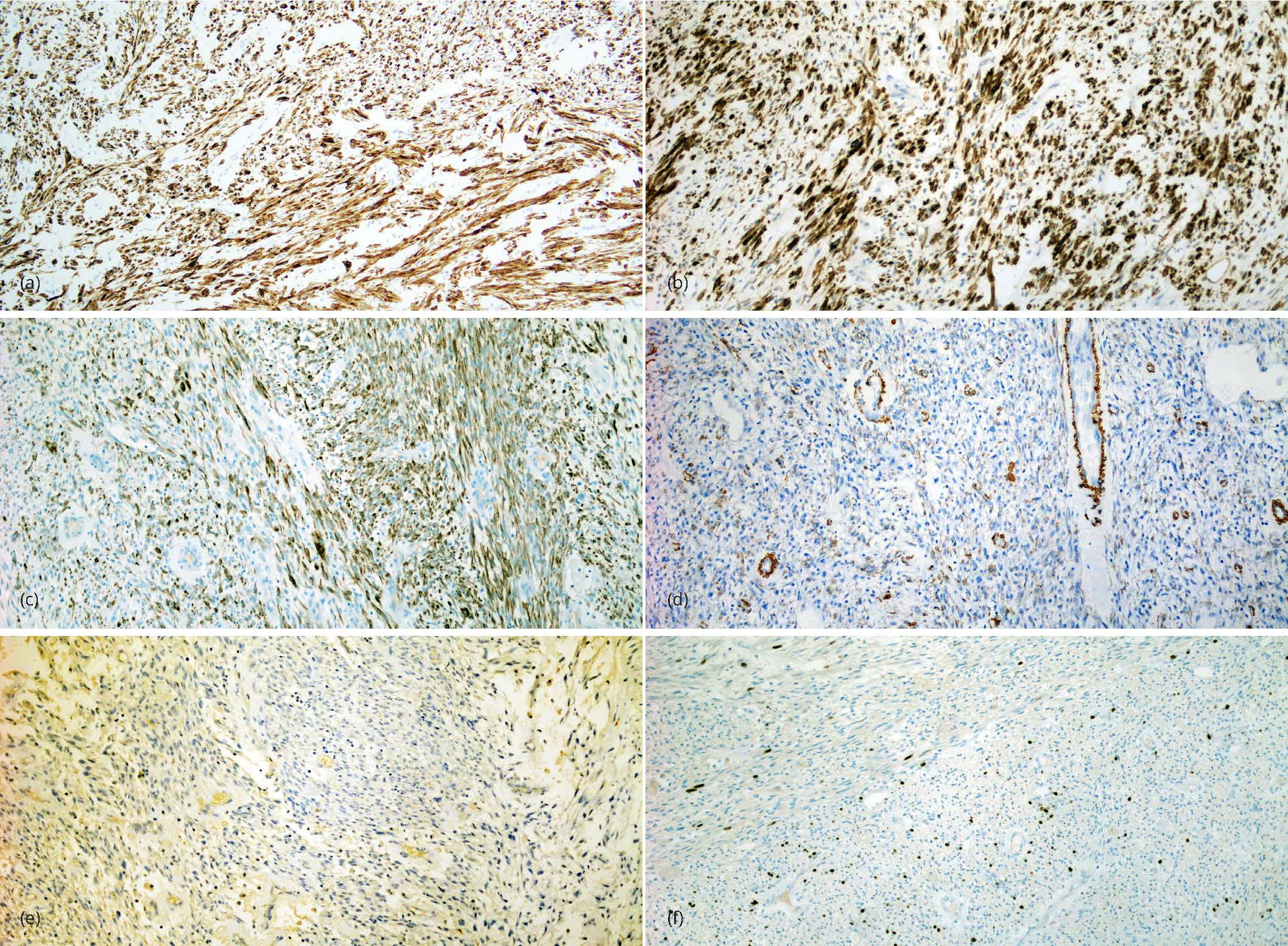
DiscussionTop
IMTs are mesenchymal neoplasms that were first described in the lung by Bahadori and Liebowin in 1973 as inflammatory pseudotumor, plasma cell granuloma, xanthomatous pseudotumor, xanthoma and histiocytoma [5, 6]. The recent documented cytogenetic abnormalities in these tumors strongly favor their neoplastic nature, and hence should be distinguished from other pseudotumors or other reactive inflammatory lesions. Currently they are considered true neoplasms with intermediate biological potential and indolent clinical course [2-4]. Primary sites include lungs, omentum, peritoneum and mesentery [7]. Infrequently IMTs were reported in other sites including head and neck region, central nervous system, genitourinary and gynecological tract. IMT of the uterus is an under-recognized entity with less than 75 cases reported in the world literature [2, 4, 8].
About 50% the non-uterine IMTs cases have a translocation involving the ALK gene on chromosome 2p23, leading to overexpression and hyperactivation of this receptor tyrosine kinase [3, 9, 10]. That number approaches 100% of uterine IMTs [1, 4, 8, 11, 12]. ALK is a transmembrane receptor tyrosine kinase, which after dimerization and phosphorylation activates other proteins and takes part in cellular growth and maturation. At least 7 translocations involving ALK have been identified in IMT. Mutation in ALK locus in 2p23 results in fusion of the 3’ kinase portion of the ALK to the 5’portion of the partner gene in case of IMTs [7]. The presence of ALK gene alteration makes these tumors amenable to therapy with tyrosine kinase inhibitors such as crizotinib [3], particularly in patients with unresectable and/or recurrent tumors [6]. In ALK negative IMTs presence of ROS 1 and PDGFRβ kinase fusions has been detected and together ALK, ROS1 and PDGFRβ kinase fusions are identified in about 85% of IMTs cases [13]. TPM3, SEC31, IGFBP5, TIMP3, THBS1 and DES are multiple ALK fusion partner genes that have been also reported in IMTs [3]. Recent studies described novel fusions involving ROS1, platelet-derived growth factor receptor betas and ETS translocation variant (ETV6) genes in a subset of ALK negative patients [2, 3, 10]. ALK positive tumors have two types of staining patterns; a diffuse granular cytoplasmic staining pattern without perinuclear accentuation and a diffuse granular cytoplasmic staining pattern with perinuclear accentuation [14].
Clinically IMTs occur across a large age range from 6-78 years with a mean of 41 years [6, 8, 11]. Reports have shown no relationship to hormonal use or prior pelvic surgery. Patients seek medical advice for a variety of reasons including mass-associated symptoms such as abdominal discomfort, fullness, pain and dyspareunia, menstrual irregularities with menorrhagia and severe anemia on occasions and constitutional symptoms as fever, malaise, weight loss, leukocytosis, and elevated c-reactive proteins and sedimentation rare [8, 11, 15, 16]. Their presence is associated strongly with the detection of IL-6 and a fall in IL-6 levels was noted after tumor excision [15]. They are more commonly reported in pregnant women, which is attributed to the strong estrogen and progesterone receptor positivity of the tumor [2, 4, 8, 10]. In a review of 72 cases reported in the literature, 65 (90.3%) occurred in the uterine corpus [1]. Rare cases have been reported in the cervix and two cases in the placenta [17]. Grossly, IMTs are fleshy neoplasms with a whorled appearance. Infiltration may also be seen as a jagged sawtooth appearance or percolation of tumor cells into the myometrium [2-4, 8, 10]. Uterine IMTs are seen as submucosal polypoid masses in half of the cases, other forms seen as discrete, intramural, transmural or subserosal masses as tan pink or white with interspersed yellow areas and firm to fleshy to soft and gelatinous/ mucinous/ myxoid consistency [2, 3, 8]. Hemorrhage, cystic change and necrosis are commonly seen. Tumor size ranges from 1.5 to 20 cm [2, 3, 8]. Classically they are spindle cell neoplasms with three histologic patterns including fascicular/ hypercellular, myxoid/ hypocellular and hyalinized patterns [4, 8, 10]. Usually there is an associated brisk lymphoplasmacytic inflammatory infiltrate. Presence of a lymphoplasmacytic infiltrate should encourage the pathologist to perform immunohistochemical staining [18]. Classic IMTs have a tissue culture-like appearance, low mitotic activity and no or minimal cytologic atypia [8, 10].
Myxoid differentiation is a common phenomenon that is seen in a wide range of mesenchymal lesions. In the uterus, myxoid differentiation of smooth muscle neoplasm is not uncommon and is well characterized [2]. In addition to smooth muscle neoplasms, there is a spectrum of other tumors with mesenchymal features albeit rare that exist including endometrial stromal neoplasms, endometrial undifferentitated/ dedifferentiated carcinoma and biphasic tumors such as carcinosarcoma and adenosarcomas [2, 12]. Distinguishing tumors such as IMTs or myxoid high grade endometrial stromal sarcoma from myxoid leiomyosarcoma is critically important. Frequently these tumors have overlapping morphologic and immunohistochemical features. When encountered, they pose a diagnostic challenge. Pathologists need to be aware of the different identities and need to familiarize themselves with their clinical, morphological, immunohistochemical and molecular features for appropriate management and treatment. The morphologic and immunohistochemical features of IMTs requires careful histologic evaluation with a battery of stains including ALK [2, 3, 19]. When ALK is negative, additional studies should be performed to exclude ALK negative IMTs before subjecting patients to further testing and treatment for the other mimickers. Distinction of IMT from conventional leiomyosarcoma can be made based on low mitotic index, absence of atypical mitotic figures or significant nuclear atypia and absence of coagulative necrosis. Distinction of IMT from myxoid leiomyosarcoma is difficult however, as the latter is known to have low mitotic index but an aggressive course. In such cases, the key to differentiating diagnostic features are infiltrative microscopic pattern of myxoid leiomyosarcoma, where irregular tongues of tumor invade the adjacent tissues and vascular invasion is often seen [2]. In contrast, uterine IMTs have round pushing borders without any signs of invasion. Lymphoplasmacytic infiltrate is a feature that is reported in uterine IMTs and absent in myxoid leiomyosarcoma [8].
In general, the gold standard treatment for such tumors is complete surgical excision. Mini invasive surgery is another option to be considered in young patients [1]. Because there is a propensity for local recurrence with such tumors, close follow up is recommended. IMTs are best categorized as tumors of intermediate malignant potential. Effective management might require systemic chemotherapy in addition to surgery however, they usually don’t respond to these conventional therapies and might need targeted therapy with tyrosine kinase inhibitors. Parra-Herran et al. studied several patients with aggressive IMTs. Patients with adverse outcomes were noted to be older with larger tumors. Microscopically, myxoid pattern was predominant in aggressive tumors and also the borders were infiltrating into the surrounding tissue. Mitotic index was observed to be higher in cases with recurrence or metastases [4]. A case of IMT with complete regression over a course of two years was reported, the tumor showed a regression from periphery towards uterus, in a manner called “rewind backwards” when given a short course of antibiotics [20].
ConclusionTop
IMT of the uterus might “STUMP” you. Be careful. The distinction between IMTs and other mesenchymal uterine tumors can be subtle. A low threshold for ALK immunohistochemical staining is required. Correlation of gross appearance with tumor morphology is critical and it is important to extensively sample all mesenchymal tumors of the uterus.
Conflicts of interest
Authors declare no conflicts of interest.
ReferencesTop
[1]Mandato VD, Valli R, Mastrofilippo V, Bisagni A, Aguzzoli L, et al. Uterine inflammatory myofibroblastic tumor: More common than expected: Case report and review. Medicine (Baltimore). 2017; 96(48):e8974.Article Pubmed
[2]Pickett JL, Chou A, Andrici JA, Clarkson A, Sioson L, et al. Inflammatory myofibroblastic tumors of the female genital tract are under-recognized: a low threshold for ALK immunohistochemistry is required. Am J Surg Pathol. 2017; 41:1433–1442.Article Pubmed
[3]Shukla PS, Mittal. Inflammatory myofibroblastic tumor in female genital tract. Arch Pathol Lab Med. 2018.Article Pubmed
[4]Parra-Herran C, Quick CM, Howitt BE, Dal Cin P, Quade BJ, et al. Inflammatory myofibroblastic tumor of the uterus: clinical and pathologic review of 10 cases including a subset with aggressive clinical course. Am J Surg Pathol. 2015; 39(2):157–168.Article Pubmed
[5]Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973; 31:191–208.Article Pubmed
[6]Fraggetta F, Doglioni C, Scollo P, Pecciarini L, Ippolito M, et al. Uterine inflammatory myofibroblastic tumor in a 10-year-old girl presenting as polypoid mass. J Clin Oncol. 2015; 33:e7–e10.Article Pubmed
[7]Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA. Inflammatory myofibroblastic tumor of the uterus: A clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am J Surg Pathol. 2005; 29(10):1348–1355.Article Pubmed
[8]Mohammad N, Haimes JD, Mishkin S, Kudlow BA, Leong MY, et al. ALK is a specific diagnostic marker for inflammatory myofibroblastic tumor of the uterus. Am J Surg Pathol. 2018; 42(10):1353–1359.Article Pubmed
[9]Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001; 14(6):569–576.Article Pubmed
[10]Bennett JA, Nardi V, Rouzbahman M, Morales-Oyarvide V, Nielsen GP, et al. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol. 2017; 30(10):1489–1503.Article Pubmed
[11]Fuehrer NE, Keeney GL, Ketterling RP, Knudson RA, Bell DA. ALK-1 protein expression and ALK gene rearrangements aid in the diagnosis of inflammatory myofibroblastic tumors of the female genital tract. Arch Pathol Lab Med. 2012; 136; 623–626.Article Pubmed
[12]Subbiah V, McMahon C, Patel S, Zinner R, Silva EG, et al. STUMP un"stumped": Anti-tumor response to anaplastic lymphoma kinase (ALK) inhibitor based targeted therapy in uterine inflammatory myofibroblastic tumor with myxoid features harboring DCTN1-ALK fusion. J Hematol Oncol. 2015; 8:66.Article Pubmed
[13]Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer discov. 2014; 4(8):889–895.Article Pubmed
[14]Devereaux KA, Kunder CA, Longacre TA. ALK-rearranged tumors are highly enriched in the STUMP subcategory of uterine tumors. Am J Surg Pathol. 2018.Article Pubmed
[15]Azuno Y, Yaga K, Suehiro Y, Ariyama S, Oga A. Inflammatory myoblastic tumor of the uterus and interleukin-6. Am J Obstet Gynecol. 2003; 189(3):890–891.Article Pubmed
[16]Fraggetta F, Doglioni C, Scollo P, Pecciarini L, Ippolito M, et al. Uterine inflammatory myofibroblastic tumor in a 10-year-old girl presenting as polypoid mass. J Clin Oncol. 2015; 33(2):e7–e10.Article Pubmed
[17]Schoolmeester JK, Sukov WR. ALK-rearranged inflammatory myofibroblastic tumor of the placenta, with observations on site of origin. Int J Gynecol Pathol. 2017; 36(3):228–229.Article Pubmed
[18]Oliva E. Practical issues in uterine pathology from banal to bewildering: The remarkable spectrum of smooth muscle neoplasia. Mod Pathol. 2016; 29 Suppl 1:S104–120.Article Pubmed
[19]Parra-Herran C, Schoolmeester JK, Yuan L, Dal Cin P, Fletcher CD, et al. Myxoid Leiomyosarcoma of the uterus: A clinicopathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am J Surg Pathol. 2016; 40(3):285–301.Article Pubmed
[20]Markovic Vasiljkovic B, Plesinac Karapandzic V, Pejcic T, Djuric Stefanovic A, Milosevic Z, et al. Follow-up imaging of inflammatory myofibroblastic tumor of the uterus and its spontaneous regression. Iran J Radiol. 2016; 13(1):e12991.Article Pubmed



