Journal of Operative and Esthetic Dentistry
An International Peer-Reviewed Open Access Journal
ISSN 2398-029X


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Operative and Esthetic Dentistry
Volume 2, Issue 1, March 2017, Pages 1–7
Original researchOpen Access
Effect of adhesive filler content on marginal adaptation of class II composite resin restorations
- 1 Department of Operative Dentistry, Université Laval, Quebec City, Quebec, G1V 0A6, Canada
- 2 Bisco, Schaumburg, IL, 60193, USA
- 3 Department of Preventive and Community Dentistry, University of Iowa, Iowa City, IA, 52241, USA
- 4 Department of Family Dentistry, University of Iowa, Iowa City, IA, 52241, USA
*Corresponding author: Dr. Laurie St-Pierre, Department of Operative Dentistry, Université Laval, Quebec City, Quebec, G1V 0A6, Canada. Tel.: 418-656-2131, ext. 7624; Fax: 418-656-2720; E-mail: laurie.st-pierre@fmd.ulaval.ca
Received 10 January 2017 Revised 12 February 2017 Accepted 17 February 2017 Published 27 February 2017
DOI: http://dx.doi.org/10.14312/2398-029X.2017-1
Copyright: © 2017 St-Pierre L, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Introduction: Adhesive formulation may affect the marginal adaptation of composite resin restorations. Objectives: To evaluate the effect of adhesive filler content on the gingival marginal adaptation of Class II composite resin restorations. Materials and methods: Class II cavity preparations were made in ninety intact extracted human molars. Specimens were randomized and equally distributed in 6 groups (n = 15). Preparations were etched and All-Bond 3 primers A + B (Bisco, Schaumburg, IL/USA) were applied. One group received All-Bond 3 adhesive/resin (50 wt% filler) and the other five groups received the same resin composition but with filler contents of 0, 10, 20, 30 and 40 wt%. Teeth were restored with Filtek Supreme Ultra (3M, St-Paul, MN/USA), finished and stored in artificial saliva (37°C/24 h) before replicas were made for FE-SEM observation (200X). Quantitative margin analysis was performed based on a four criteria: MQ1 (continuous margin), MQ2 (marginal irregularities, porosities, roughness, no gap), MQ3 (small gaps of up to 2 microns) and MQ4 (severe gaps). Data were analyzed with a one-way ANOVA based on ranked data followed by the post-hoc Bonferroni test. Results: There was no statistically significant difference in “continuous margin” observed among the six filler contents (p = 0.0886). However, significantly less severe gaps were obtained with filler levels of 30 wt% and 40 wt% compared to 0 wt% (p = 0.0159). A noticeable but non-significant difference was observed for the unfilled adhesive (0 wt%), which showed the lowest mean % for continuous margins and the highest for severe gaps. Conclusion: Filler addition to adhesive appears to improve the marginal adaptation. Filler contents of 30 wt% and 40 wt% significantly reduced the occurrence of severe gaps compared to 0 wt%.
Keywords: adhesive; filler; particles; marginal adaptation; composite; Class II restorations
IntroductionTop
Clinical performance of dental adhesives has greatly improved since their introduction to dentistry. Different formulations and methods of application are currently available to the dental practitioner. However, research in dental adhesion is constantly seeking for ways to enhance the longevity and effectiveness of bonded restorations.
Marginal adaptation of composite resin restorations, particularly at the gingival margin of Class IIs, is still a major concern. Marginal defects such as gaps increase the risks of post-operative sensitivity [1] and recurrent caries, the latter being reported as one of the main causes of failure of composite resin restorations [2-4] especially with high caries risk patients [5]. Therefore, marginal integrity and seal are of primary importance for the clinical success of bonded restorations.
In this regard, one factor influencing the marginal seal of composite resin restorations relies on the effectiveness and properties of the adhesive layer [6]. In order to improve the radiopacity, mechanical properties and durability, manufacturers have added filler particles to the adhesive composition [7]. The addition of filler particles increases the adhesive viscosity and allows the placement of a thicker layer which has been suggested to act as a shock-absorbing layer or an elastic intermediate layer to help resisting polymerization shrinkage and preserving marginal integrity [8-13]. This has been described as the elastic cavity wall theory [10]. In addition, filler particles are expected to improve the bond strength by reinforcing the hybrid layer [14, 15] and, depending on the type of filler particle, to generate a radiopaque adhesive layer [1, 2, 16, 17].
However, an increased adhesive viscosity and filler particle size above the collagen interfibrillar space of approximately 20 nm may prevent a proper infiltration of the adhesive in the collagen fiber network, resulting in a deficient hybrid layer containing internal voids and cracks which may decrease the bond strength [7, 9, 16, 8-21]. Also, fillers may form clusters and further obstruct adhesive penetration within the collagen network [9, 22].
Studies comparing bond strength of filled and unfilled adhesives have provided controversial results on the potential benefits of filler particles. Mirmohammadi et al. [23] did not find a significant difference in microleakage between a regularly filled (10%) and unfilled version of Clearfil SE Bond. Contrarily, Miyazaki et al. [7] obtained maximal shear bond strength with 10 wt% filler content, which gradually decreased above 20 wt%. Gallo et al. [14] stated that the shear bond strength of filled adhesives is statistically comparable or greater than unfilled adhesives whereas Fanning et al. [12] suggested a noticeable but non-significant difference in shear bond strength between filled and unfilled adhesives. Some other bond strength studies, microtensile [24] and inverted cone tensile bond strength [25], reported no difference between filled and unfilled adhesives, meanwhile, Kasraei et al. [9] reported an increase in microtensile bond strength with an incorporation of nanofiller of up to 1%. One study conducted by Tani et al. [6] found that adding filler particles increased the tensile bond strength, but compromised the marginal seal compared to unfilled adhesive. One study from Martins et al. [26] concluded that adding barium-borosilicate glass filler of up to 50% appeared to decrease the adhesive solubility without compromising its mechanical properties. The improvement in mechanical properties with the addition of filler particles seems to be product related [27] and may depend on other factors such as filler size, type of adhesive and its composition [9, 14, 19, 28].
Up to date, it is still unclear whether filler particles added to dental adhesives have an effect on the marginal adaptation of composite resin restorations and what is the optimal filler ratio. Moreover, most studies evaluating the influence of filler particles are based on bond strength. Therefore the purpose of this study was to evaluate the effect of filler particles added to dental adhesive on the marginal adaptation and which filler content will most preserve the marginal integrity at the gingival margins of Class II composite resin restorations. The marginal adaptation at the tooth-restoration interface was investigated with a field emission scanning electron microscope (FE-SEM). The null hypothesis tested is that there is no difference in the marginal adaptation of Class II resin composite restorations with various amounts.
Materials and methodsTop
Ninety caries-free human molars within six months of extraction were collected from the department of Oral Surgery at the University of Iowa, in accordance with the institutional review board. Teeth were first stored in a solution of 0.2% thymol until needed and subsequently cleaned and kept at 4oC in a solution of 0.5% chloramine T for at least 24 h. Thymol and chloramine T were used in this study to store and disinfect teeth with minimal effect on tooth structures. These two storage media have been reported in the literature to have no significant effect on bond strength and microleakage [29-31]. Teeth were removed form the chloramine T and kept in artificial saliva throughout the study.
Occlusal surfaces were grounded flat to remove the cusps and to ensure that the distance from the light guide to the gingival margins was equal for all specimens. Individualized silicon putty matrices covering the circumference of the teeth were fabricated to seal the margins while restoring with composite resin. The silicon putty matrix was reduced down using silicon carbide paper 120-grit on a polishing machine (Rotopol-V, Struers, Cleveland OH, USA) until it reached the same level as the tooth structure on the occlusal surface.
Five experimental adhesive formulations were prepared by the manufacturer (Bisco, Schaumburg, IL, USA), based on All-Bond 3 adhesive/resin (Bisco), a three-step total-etch adhesive system. Specifically, the inorganic fillers of All-Bond 3 adhesive/resin (mixture of ∼50%wt of 0.7 micron barium glass particles, ∼50%wt of 40nm ytterbium fluoride particles and trace amount of silica) were mixed with the organic matrix of All-Bond 3 adhesive/resin with different levels of filler: 0, 10, 20, 30 and 40 wt%, and stored in an opaque sealed container until use. All-Bond 3 adhesive/resin contains about 50 wt% fillers (Table 1).
| Product | Compositions |
| All-bond 3 primer | Part A: Ethanol, NTG-GMA salt |
| Part B: BisGMA, BPDM, photoinitiator | |
| All-bond 3 adhesive/resin | BisGMA, UDMA, TEGDMA, silanized glass fillers (< 1 µm), ytterbium fluoride (< 100 nm) |
Class II cavity preparations (5 mm deep occluso-gingivally X 4 mm wide bucco-lingually X 2 mm deep mesio-distally) were made on either the mesial or the distal surface depending upon which offered the flattest surface and were measured with a digital caliper. The gingival margin was placed 1 mm below the cemento-enamel junction using a flat-end diamond bur in a high speed handpiece with water coolant. The occlusal surface was further flattened until a depth of 5 mm occluso-gingivally was obtained. Preparations had 90o cavosurface angles and were centered on the mesial or the distal surface of each tooth. Gingival margins were observed under a light microscope (Zeiss, Thornwood, NY, USA) at a magnification of 20X to ensure that margins were straight and well defined.
Specimens were randomized into six groups (n = 15) using a random sequence generator according to the five experimental adhesives and All-Bond 3 adhesive resin.
The silicon putty matrix was placed around the specimens to serve the function of a proximal matrix. Cavity preparations were etched with 35% phosphoric acid gel (Ultra-ecth, Ultradent, South Jordan, UT, USA). The etchant was applied to enamel first for 10 seconds and then to dentin for an additional 10 seconds. Specimens were rinsed for 20 seconds with air and water spray and blotted dried to obtain a slightly moist surface. All-Bond 3 primer (Table 1), an equal amount of part A (lot number 1100010921) and part B (lot number 1100010922) mixed, was applied for all the groups according to manufacturer’s instructions. The experimental adhesives and All-Bond 3 adhesive resin (lot number 1100010164) were well shaken before use, applied in a thin layer and excess removed with a microbrush applicator and light cured for 20 seconds (Optilux 500, Demetron, Kerr, Danbury, CT, USA). The time for adhesion procedure was monitored with a chronometer (Traceable timer, Control Company, Friendswood, TX, USA).
A 1-mm increment of composite resin Filtek Supreme Ultra (3M, St-Paul, MN, USA) shade A2B was placed horizontally at the gingival margin followed by two additional 2 mm increments. Each increment was light cured for 40 sec. The curing light intensity was verified periodically throughout the experiment to ensure an intensity of at least 600 mW/cm2.
After withdrawal of the silicon matrix, visible overhang were carefully removed using a #12 scalpel blade and the margins were finished using Sof-Lex XT discs (3M, St-Paul, MN, USA) medium- and fine-grit. Gingival margins were inspected under a light microscope at a magnification of 20X to ensure that no flash remained.
Specimens were stored in artificial saliva in a bacteriological oven at 37°C for 24 h before the fabrication of replicas. To remove debris and contamination at the tooth-restoration interface, specimens were placed in 70% ethanol in an ultrasonic bath for 2 min, rinsed and dried. Two sets of impressions were immediately obtained using a low viscosity polyvinyl siloxane (Aquasil XLV Ultra, fast set, Dentsply Caulk, Milford, DE, USA). The first impression was taken in order to further remove any contaminant at the margin and was discarded. The second impression using the same light viscosity impression material was taken, visually inspected for any imperfection and placed on a double sided tape in a sealed plastic container for at least 24 h to allow any gas formation resulting from the impression material polymerization to escape. Impressions were then poured with epoxy resin (Epoxicure, Buehler Ltd, Lake Bluff, IL, USA) and allowed to set undisturbed for 24 h in a fume hood.
Replicas were mounted on aluminum stubs using carbon tape and graphite colloidal paint, sputter coated with gold and palladium (Emitech K550, Ashford, Kent, UK) at 10 mA for 2.5 min and observed with a FE-SEM (Hitachi S-4800, Hitachi High Technologies America Inc., Pleasanton, CA, USA) at a magnification of 200X. Approximately seven to ten micrographs were taken on each replica at the gingival margin and subsequently merged together using Photoshop Elements 10 (Adobe Systems Inc., San Jose, CA, USA). Merged images were assigned to a random number to blind the examiner during measurements.
Marginal adaptation was evaluated with quantitative margin analysis. For each gingival margin, the total length of the margin (approximately 4 mm) was first measured using ImageJ software (ImageJ 1.44p, Wayne Rasband, National Institute of Health, USA). The length of any artifact such as bubbles or contamination were then measured and subtracted from the total gingival margin length. Each defect present at the adhesive interface was ranked qualitatively based on four marginal quality criteria (MQ1, MQ2, MQ3 and MQ4) previously defined by Blunck et al. [32, 33] (Table 2 and Figure 1) and its length was subsequently measured. Calculations were then made to determine the percentage of each marginal quality criterion present at the gingival margin.
| Marginal quality | Definition |
| MQ1 | Continuous margin corresponding to a margin not or hardly visible with no or slight marginal irregularities. No gap |
| MQ2 | Severe marginal irregularities*. No gap |
| MQ3 | Hairline crack or small gap of up to 2 mm in width |
| MQ4 | Severe gap (more than 2 mm in width ) |
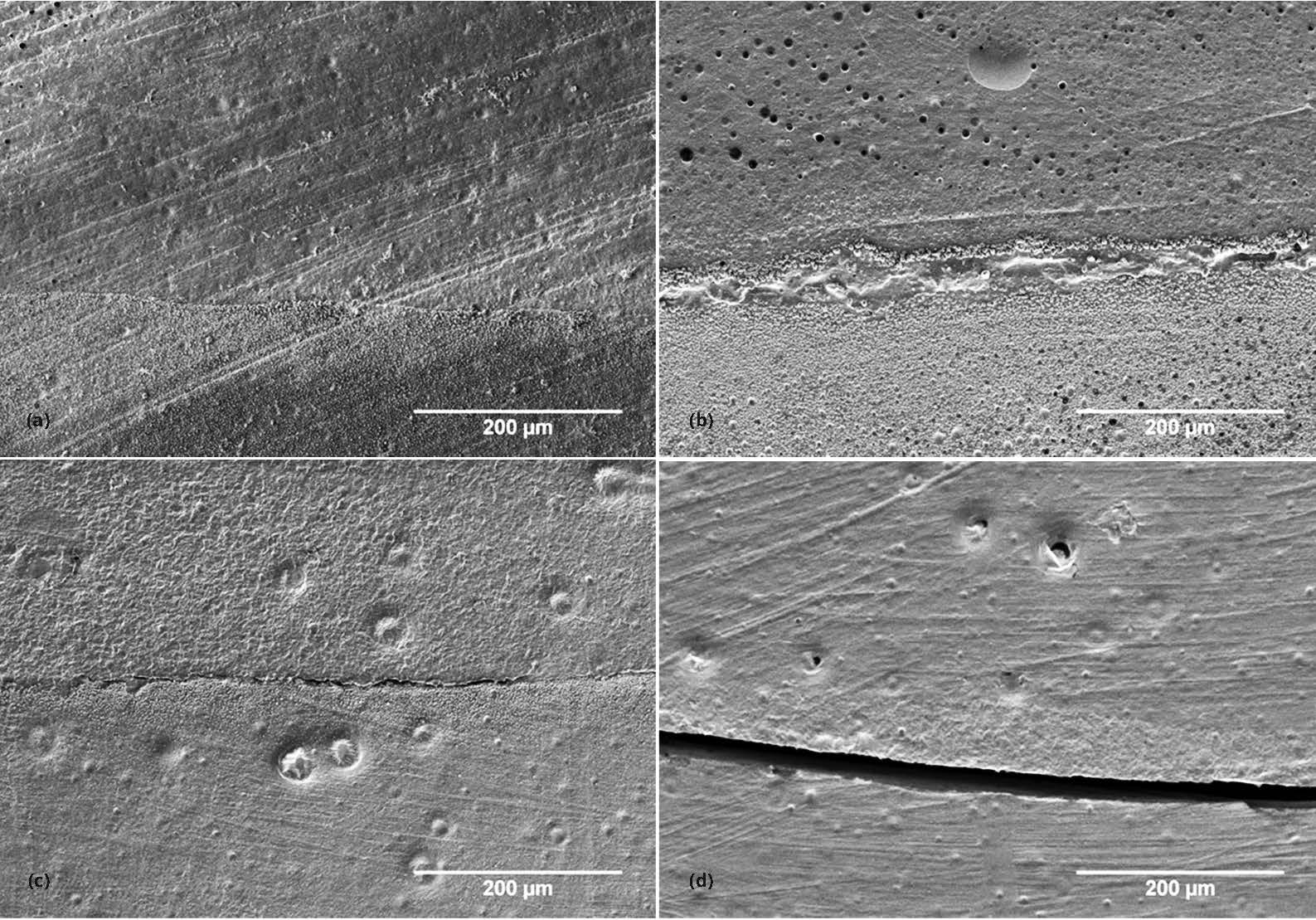
Due to the lack of normality, a one-way ANOVA based on ranked data, an equivalent test statistic to the nonparametric Kruskal-Wallis test, was used for comparison of the marginal adaptation between the different filler contents followed by the post-hoc Bonferroni multiple comparison test. SAS for Windows (v9.3, SAS Institute Inc, Cary, NC, USA) was used for the data analysis and an alpha of less than 0.05 was used as a criterion for statistical significance.
ResultsTop
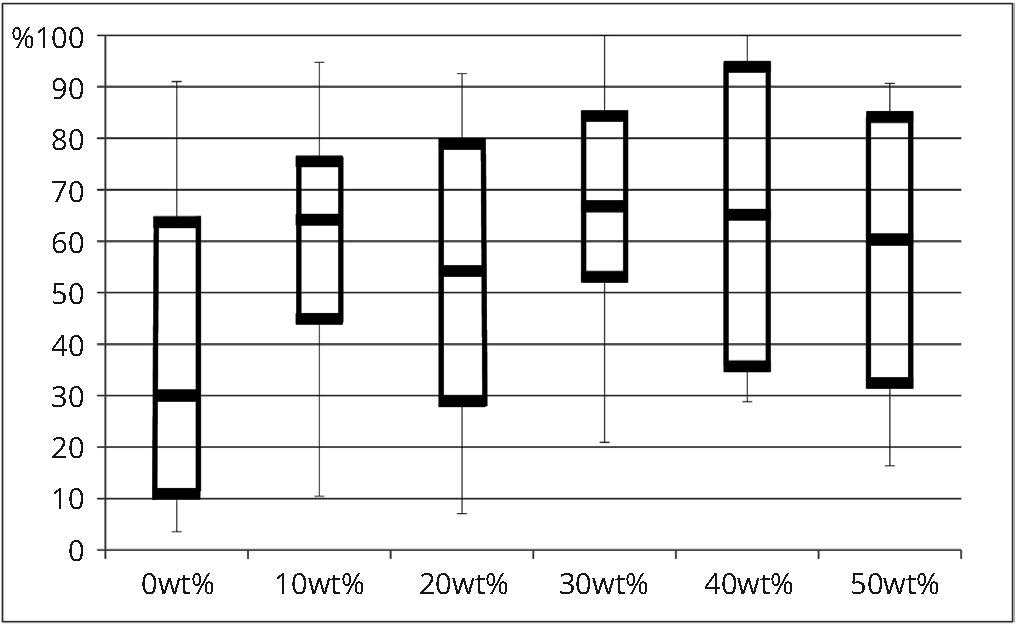
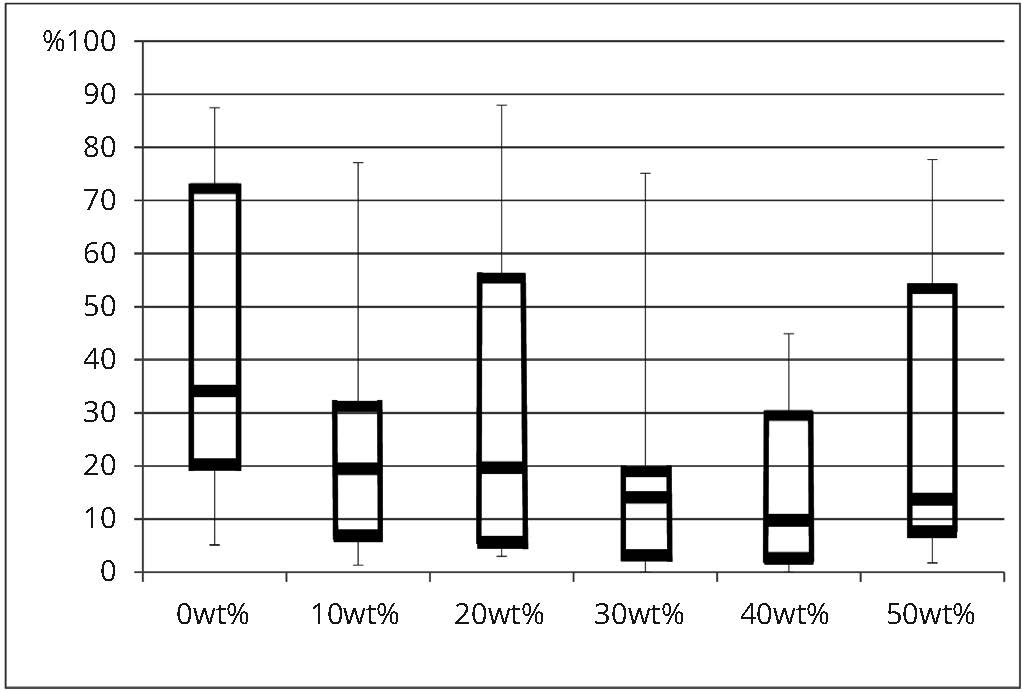
The mean percentages of each marginal quality criterion are presented in table 3 and comparisons among experimental adhesives for continuous margins and severe gaps are illustrated in figures 2 and 3. Results of a one-way ANOVA based on ranked data revealed that the filler content has no significant effect for “continuous margin” (MQ1) (F(5, 84) = 1.69; p = 0.0886), “severe marginal irregularities” (MQ2) (F(5, 84) = 0.24; p = 0.9418) and “gap of less than 2 microns” (MQ3) (F(5, 84) = 1.46; p = 0.2101) among the six filler contents tested. However, the filler content has a significant effect for “severe gap” (MQ4) (F(5, 84) = 2.98; p = 0.0159). The post-hoc Bonferroni test indicated that significantly less severe gaps were observed with filler contents of 30 wt% and 40 wt% compared to 0 wt%, but no significant differences were found among 0, 10, 20 and 50 wt% as well as among 10, 20, 30, 40 and 50 wt% (Table 3). Nonetheless, even with a lack of statistical significance, mean percentage of continuous margin for 0 wt% filler content shows the least amount of perfect margins, which starts increasing with the addition of filler (Table 3). Mean percentage of severe gaps follows the same trend where the higher percentage of severe gaps was observed within 0 wt% filler compared to all the other groups.
| Filler level | N | MQ1** | MQ2** | MQ3** | MQ4** | ||||
| Mean % (SD) | Median | Mean % (SD) | Median | Mean % (SD) | Median | Mean % (SD) | Median | ||
| 0 wt% | 15 | 39.36 (30.74)A | 30.02 | 6.75 (6.89) A | 6.33 | 9.81 (11.34) A | 5.88 | 44.08 (29.09) A | 34.15 |
| 10 wt% | 15 | 60.27 (22.15) A | 64.17 | 7.49 (6.29) A | 5.45 | 9.62 (7.72) A | 7.04 | 22.62 (19.57) A, B | 19.48 |
| 20 wt% | 15 | 54.25 (27.94) A | 54.23 | 7.22 (6.15) A | 8.56 | 5.65 (4.45) A | 3.95 | 32.88 (27.95) A, B | 19.69 |
| 30 wt% | 15 | 68.02 (22.33) A | 66.8 | 9.77 (8.94) A | 8.31 | 5.67 (9.76) A | 1.99 | 16.54 (18.19) B | 14.1 |
| 40 wt% | 15 | 66.19 (24.86) A | 65.18 | 10.67 (11.20) A | 7.25 | 7.76 (8.64) A | 6.08 | 15.38 (15.52) B | 9.8 |
| 50 wt% | 15 | 56.26 (25.87) A | 60.33 | 8.22 (6.55) A | 6.06 | 7.9 (8.23) A | 5.42 | 27.62 (26.58) A, B | 13.77 |
DiscussionTop
This study evaluated the effect of the filler content in five experimental adhesives and the commercially available 50 wt% filled All-Bond 3 on the marginal adaptation at the gingival margin of Class II composite resin restorations.
It has been suggested that adding filler particles to adhesive would increase its mechanical properties and the bond strength of composite resin restorations [8, 9, 11, 12, 15]. However, there is a lack of scientific evidence whether filler particles enhance the restoration outcome. Furthermore, the optimal filler level to be added to the adhesive to improve its properties remains unknown.
Most studies comparing the effect of filler particles in adhesive evaluated two-step total-etch or self-etch adhesives and comparisons with such studies need to be done with caution [7, 9, 19]. All-Bond 3 is a dual-cured, three-step total-etch adhesive system. In such system, enamel and dentin are first etched to remove the smear layer, create micromechanical retention in enamel, open dentinal tubules and decalcify the intertubular and peritubular dentin leaving a network of collagen fibers. A primer is then first actively applied with scrubbing motion in order to properly and completely infiltrates this collagen network to enhance the wettability of the dentin and to allow a better infiltration of the adhesive resin which forms the hybrid layer [34]. The bond strength depends on the complete infiltration and replacement of the dissolved dentin by a polymerized resin [34]. Although filler particles are added in some adhesives to improve their mechanical properties, they have been reported to potentially prevent the proper infiltration of the adhesive in the collagen network and create a deficient hybrid layer [7, 9, 16, 18, 19, 23].
In the present study, only Filtek Supreme Ultra (3M) was used as a composite resin and all specimens were subjected to the same treatment as closely as possible to intra-oral conditions so that the filler content would be the only variable. The mean percentage values show a tendency for the addition of filler particles to improve the marginal adaptation even though no statistically significant differences on the occurrence of “continuous margins” (marginal quality criterion 1), “marginal irregularities” (marginal quality criterion 2) and “small gaps of up to 2 microns” (marginal quality criterion 3) were found between the filler levels tested. However, significantly less “severe gaps” (marginal quality criterion 4) were observed with filler contents of 30 wt% and 40 wt% compared to 0 wt%. Compared to the commercially available 50 wt% filled All-Bond 3, no significant differences were found between the different filler levels tested for all marginal quality criteria although the mean percentage values showed a tendency for fewer “severe gaps” with filler contents of 30 wt% and 40 wt% and for more “severe gaps” with filler levels of 0 wt% and 20 wt%. This is in accordance with the study of Martins et al. [35], which reported that the addition of 30 wt% barium-borosilicate glass did not compromised the bond strength.
While preparing the specimens, an increase in the adhesive viscosity was gradually noticed proportionally with the filler level. The higher proportion of severe gaps within the unfilled, 0 wt%, adhesive group could be due to the low viscosity of the adhesive, which creates an ultra thin adhesive layer that may not be sufficient to resist the stress generated during the polymerization of the composite resin. Moreover, thin adhesive layers may not be completely polymerized due to the oxygen inhibited layer [36]. On the other hand, the high viscosity of the adhesive with a filler level of 50 wt% may have prevented a good infiltration of the resin between the exposed collagen fibers.
Nonetheless, with their higher viscosity, filled adhesives can be placed in a thicker layer, more flexible than the overlying composite resin, which may work as an elastic intermediate layer and absorb stresses produced during the composite resin polymerization preserving marginal integrity [8, 12]. However, fillers increase the elastic modulus and stiffness of the adhesive, which reduce its ability to act as a stress buffer [17]. This phenomenon was most likely not in cause in the study with filler level of 50 wt% or less, but could have been observed with higher percentage of filler content.
In addition, a thicker layer may not be uniform especially at the line angles where the adhesive is subjected to pooling due to gravity and becomes thin at the margin. This phenomenon was observed in a study conducted by Choi et al. [11] where the results suggested higher concentration of stresses in thin area such as at the margins leading to a gap formation at the tooth-restoration interface. Accumulation of adhesive at the line angles was also observed in our study mainly with higher filler contents. However, because of the presence of the putty matrix, some pooling may also have occurred along the margin leading to a relatively thick adhesive layer at the margin and diminishing the stress concentration.
Filler particle size above the collagen interfibrillar space of approximately 20 nm has been suggested to be another factor that may affect the marginal adaptation by preventing a proper infiltration of the collagen fiber network [7, 9, 16, 18, 19, 23]. In the present study, the fillers consist of a mixture of ∼50%wt of 0.7 micron barium glass particles, 50%wt of 40 nm ytterbium fluoride particles and trace amount of silica. Barium glass and ytterbium fluoride particles are wider than the estimated interfibrillar space dimension. With low percentages of filler, the adhesive resin might still infiltrate the collagen matrix, but as the percentage of filler increases, these wider filler particles, in conjunction with an increased adhesive viscosity, may accumulate on top of the collagen network impeding the infiltration of the resin into the collagen network resulting in a flawed hybrid layer. In addition, filler particles might precipitate and form clusters which may preclude even more the penetration of adhesive resin [9, 22]. On the other hand, for same filler content, smaller particles increase the viscosity even more due to the increase in surface area [13] and therefore, particles may penetrate between the collagen fibers, but the higher viscosity may not allow a complete replacement of the demineralized dentin.
The degree of conversion of dental adhesives may also affect the mechanical properties and lead to bond failure [37]. For composite resins, the depth of cure has been reported in some studies to undergo a decrease of conversion with an increase in the filler content [38] which could be due to the light scattering effect of the particles [39]. While a recent study of Conde et al. [37] comparing different filler contents (1-10%) in dental adhesive did not find an effect on the degree of conversion, Miyazaki et al. [7] reported that adhesive with a filler content superior to 40 wt% may not be completely polymerized leading to a weak bond. This may in part explain the slightly higher prevalence of gaps in a filler level of 50 wt% compared to 30 wt% and 40 wt% observed in our study. More differences might have been observed with filler levels superior to 50 wt%, which were not tested in the study.
Another factor that may influence gap formation particularly in Class IIs is the C-factor. In this study, the thickness of the first increment was 1 mm, which minimized the C-factor. It is believed that a thicker layer may have further challenged the adhesive bond and may have lead to more differences between the filler levels.
The lack of significant differences between the filler levels in this study is likely due to the wide range of variability which might be attributable to the technique sensitivity of the adhesive technique used and other limitations of the study such as the finishing of the margin that was performed which may create gaps or marginal defects. On the other hand, the lack of significant difference observed in continuous margins among the groups may be the result to the good quality of the adhesive which did not allow the filler level to have an impact.
The result of our study may not apply to other materials since only All-Bond 3 was tested. Moreover, only Filtek Supreme Ultra as a composite resin was used. It is possible that a composite resin with higher elastic modulus would have shown more significant differences. Furthermore, only adhesion to dentin was evaluated and will most likely differ than that of enamel. The results may also have been different if thermal cycling tests challenging the gap formation resistance would have been performed. Another limitation of this in vitro study is the use of storage solutions of thymol and chloramine T to store and disinfect teeth before manipulations. Although some study reported no significant effect on bond strength and micro microleakage leakage when using thymol and chloramine T [29, 31] it has also been suggested that thymol, being a phenolic compound, may inhibit the polymerization of methyl methacrylate [40] and therefore may have influenced the results. Clinical limitations may also arise from a thick adhesive layer such as wear of the adhesive overtime and diagnostic issues if the particles are not radiopaque [11]. To further understand the effect of filler particles in adhesives on the marginal integrity, comparison of microtensile bond strength and mode of failure in relation to the elastic moduli among the groups tested as well as long term studies assessing the degradation of the adhesive layer and the long term thermal cycling effect in relation with the filler content would be relevant.
ConclusionTop
Within the limitation of this in vitro study, we reject the null hypothesis that there is no difference in the marginal adaptation of Class II resin composite restorations with various amounts of adhesive filler content. Furthermore, it can be concluded that adhesives containing a filler content of 30 wt% and 40 wt% showed a statistically significant reduction in severe gaps compared to the unfilled adhesive (0 wt%). Moreover, a noticeable but non-significant improvement in the marginal adaptation was observed with the addition of filler particles. This may increase the longevity of composite resin restorations often used for their esthetics and conservation of sound tooth structure.
Acknowledgements
The authors would like to thank the Central Microscopy Research Facility at the University of Iowa for the use of the field emission scanning electron microscope as well as Bisco Inc. for donating their products.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
Except for one author who is a scientist at Bisco Inc., the authors do not have any financial interest in the companies whose materials are included in this article.
ReferencesTop
[1]Brannstrom M. Communication between the oral cavity and the dental pulp associated with restorative treatment. Oper Dent. 1984; 9(2):57–68.Article Pubmed
[2]Bernardo M, Luis H, Martin M, Leroux B, Rue T, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007; 138(6):775–783.Article Pubmed
[3]Forss H, Widstrom E. Reasons for restorative therapy and the longevity of restorations in adults. Acta Odontol Scand. 2004; 62(2):82–86.Article Pubmed
[4]Rasines Alcaraz MG, Veitz-Keenan A, Sahrmann P, Schmidlin PR, Davis D, et al. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst Rev. 2014; 3:CD005620.Article Pubmed
[5]Opdam NJ, Bronkhorst EM, Loomans BA, Huysmans MC. 12-year survival of composite vs. amalgam restorations. J Dent Res. 2010; 89(10):1063–1067.Article Pubmed
[6]Tani C, Itoh K, Hisamitsu H, Wakumoto S. Effect of filler content bonding agent on bonding efficacy of 4-META MMA/TBB bonding agent. Dent Mater J. 1994; 13:131–137.Article Pubmed
[7]Miyazaki M, Hinoura K, Onose H, Moore BK. Influence of filler addition to bonding agents on shear bond strength to bovine dentin. Dent Mater. 1995; 11(4):234–238.Article Pubmed
[8]Armstrong SR, Keller JC, Boyer DB. The influence of water storage and C-factor on the dentin-resin composite microtensile bond strength and debond pathway utilizing a filled and unfilled adhesive resin. Dent Mater. 2001; 17(3):268–276.Article Pubmed
[9]Kasraei SH, Atai M, Khamverdi Z, Nejad SK. Effect of nanofiller addition to an experimental dentin adhesive on microtensile bond strength to human dentin. J Dent Tehran Univ Med Sci Kasraei. 2009; 6(2):91–96.Article Pubmed
[10]Kemp-Scholte CM, Davidson CL. Complete marginal seal of Class V resin composite restorations effected by increased flexibility. J Dent Res. 1990; 69(6):1240–1243.Article Pubmed
[11]Choi KK, Condon JR, Ferracane JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res 2000; 79(3):812–817.Article Pubmed
[12]Fanning DE, Wakefield CW, Robbins JW, Bagley AL. Effect of a filled adhesive on bond strength in three dentinal bonding systems. Gen Dent. 1995; 43(3):256–262.Pubmed
[13]Lee J, Um C, Lee I. Rheological properties of resin composites according to variations in monomer and filler composition. Dent Mater. 2006; 22(6):515–526.Article Pubmed
[14]Gallo J, Comeaux R, Haines B, Xu X, Burgess J, et al. Shear bond strength of four filled dentin bonding systems. Oper Dent. 2001; 26(1):44–47.Pubmed
[15]Belli R, Kreppel S, Petschelt A, Hornberger H, Boccaccini AR, et al. Strenthening of dental adhesives via particle reinforcement. J Mech Behav Biomed Mater. 2014; 37:100–108.Article Pubmed
[16]Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007; 28(26):3757–3785.Article Pubmed
[17]Labella R, Lambrechts P, Van Meerbeek B, Vanherle G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent Mater. 1999; 15(2):128–137.Article Pubmed
[18]Kim JS, Cho BH, Lee IB, Um CM, Lim BS, et al. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J Biomed Mater Res B Appl Biomater. 2005; 72(2):284–291.Article Pubmed
[19]Nunes M, Swift E, Perdigao J. Effects of adhesive composition on microtensile bond strength to human dentin. Am J Dent. 2001; 14(6):340–343.Pubmed
[20]Tay F, Moulding K, Pashley D. Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent. 1999; 1(2):103–117.Pubmed
[21]Watanabe I, Nakabayashi N, Pashley DH. Bonding to ground dentin by a phenyl-P self-etching primer. J Dent Res. 1994; 73(6):1212–1220.Article Pubmed
[22]Osorio E, Toledano M, Yamauti M, Osorio R. Differential nanofiller cluster formations in dental adhesive systems. Microsc Res Tech. 2012; 75(6):749–757.Article Pubmed
[23]Mirmohammadi H, Khosravi K, Kashani K, Kleverlaan C, Feilzer A. Influence of filler existence on microleakage of a self-etch adhesive system. J Conserv Dent. 2014; 17(2):175–178.Article Pubmed
[24]Can Say E, Nakajima M, Senawongse P, Soyman M, Ozer F, et al. Microtensile bond strength of a filled vs unfilled adhesive to dentin using self-etch and total-etch technique. J Dent. 2006; 34(4):283–291.Article Pubmed
[25]Lee Y, Pinzon L, O’Keefe K, Powers J. Effect of filler addition on the bonding parameters of dentin bonding adhesives bonded to human dentin. Am J Dent. 2006; 19(1):23–27.Pubmed
[26]Martins GC, Meier MM, Loguercio AD, Reis A, Gomes JC, et al. Effects of adding barium-borosilicate glass to a simplified etch-and-rinse adhesive on radiopacity and selected properties. J Adhes Dent. 2014; 16(2):107–114.Article Pubmed
[27]Giannini M, Mettenburg D, Arrais C, Rueggeberg F. The effect of filler addition on biaxial flexure strength and modulus of commercial dentin bonding systems. Quintessence Int. 2011; 42:e39–43.Article Pubmed
[28]Giannini M, Liberti M, Arrais C, Reis A, Mettenburg D, et al. Influence of filler addition, storage medium and evaluation time on biaxial flexure strength and modulus of adhesive systems. Acta Odontol Scand. 2012; 70(6):478–484.Article Pubmed
[29]Aquilino S, Williams V. The effect of storage solutions and mounting media on the bond strengths of a dentinal adhesive to dentin. Dent Mater. 1987; 3(3):131–134.Article Pubmed
[30]Haller B, Hofmann N, Klaiber B, Bloching U. Effect of storage media on microleakage of five dentin bonding agents. Dent Mater. 1993; 9(3):191–197.Article Pubmed
[31]Mobarak E, El-Badrawy W, Pashley D, Jamjoom H. Effect of pretest storage conditions of extracted teeth on their dentin bond strengths. J Prosthet Dent. 2010; 104(2):92–97.Article Pubmed
[32]Blunck U, Roulet J. In vitro marginal quality of dentin-bonded composite resins in Class V cavities. Quintessence Int 1989; 20(6):407–412.Pubmed
[33]Blunck U, Zaslansky P. Enamel margin integrity of Class I one-bottle all-in-one adhesives-based restorations. J Adhes Dent. 2011; 13(1):23–29.Article Pubmed
[34]Swift EJ, Perdigao J, Heymann H. Bonding to enamel and dentin: A brief history and state of the art. Quintessence Int. 1995; 26(2):95–110.Pubmed
[35]Martins GC, Reis A, Loguercio AD, Zander-Grande C, Meier M, et al. Does making an adhesive system radiopaque by filler addition affect its bonding properties J Adhes Dent. 2015; 17(6):513–519.Article Pubmed
[36]Frankenberger R, Lopes M, Perdigão J, Ambrose W, Rosa B. The use of flowable composites as filled adhesives. Dent Mater. 2002; 18(3):227–238.Article Pubmed
[37]Conde M, Zanchi C, Rodrigues-Junior S, Carreño N, Ogliari FA, et al. Nanofiller loading level: Influence on selected properties of an adhesive resin. J Dent. 2009; 37(5):331–335.Article Pubmed
[38]Halvorson R, Erickson R, Davidson C. The effect of filler and silane content on conversion of resin-based composite. Dent Mater. 2003; 19(4):327–333.Article Pubmed
[39]Ferracane J, Aday P, Matsumoto H, Marker V. Relationship between shade and depth of cure for light-activated dental composite resins. Dent Mater. 1986; 2(2):80–84.Article Pubmed
[40]Fujisawa S, Kadoma Y. Effect of phenolic compounds on the polymerization of methyl methacrylate. Dent Mater. 1992; 8(5):324–326.Article Pubmed



