Journal of Radiology and Imaging
An International Peer-Reviewed Open Access Journal
ISSN 2399-8172


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Radiology and Imaging
Volume 3, Issue 1, January 2018, Pages 1–5
Original researchOpen Access
Usefulness of a balloon-expandable, covered stent for the transjugular intrahepatic portosystemic shunt
- 1 University Hospital, Department of Gastroenterology, Hugstetterstrasse 55, 79106 Freiburg, Germany
- 2 Praxiszentrum fürGastroenterologie, Bertoldstrasse 48, 79098 Freiburg, Germany
- 3 University Hospital, Department of Radiology, Hugstetterstrasse 55, 79106 Freiburg, Germany
- 4 Städtisches KlinikumSaarbrücken, Winterberg 1, 66119 Saarbrücken, Germany
*Corresponding author: Martin Rössle, MD., Professor of Medicine, Praxiszentrum and University Hospital, Freiburg, Bertoldstrasse 48, 79098 Freiburg, Germany. E-mail: Martin-Roessle@t-online.de
Received 2 November 2017 Revised 11 December 2017 Accepted 19 December 2017 Published 28 December 2017
DOI: http://dx.doi.org/10.14312/2399-8172.2018-1
Copyright: © 2018 Rössle M, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
The availability of polytetrafluoroethylene (PTFE) covered, self-expandable nitinol stents in 2001 considerably improved the patency, response rates and survival of the transjugular intrahepatic portosystemic shunt (TIPS). Side effects of portosystemic shunting such as hepatic encephalopathy (HE) and worsening of hepatic function, however, remained a problem. To reduce HE, underdilatation of nitinol stents has been practiced for many years. However, as shown recently, underdilatation was a flop since, due to their intrinsic memory, nitinol stents always expanded to reach their nominal diameter of 8 or 10 mm. To overcome this problem and to be able to perform permanent shunts with a smaller diameter of < 8 mm, we studied the usefulness of a balloon-expandable, covered, metallic stent which allowed adjustment to any diameter between 5 and 12 mm. Methods: 30 patients with cirrhosis and symptomatic portal hypertension were included. The mean Child-Pugh score was 8 ± 2.17 patients had refractory ascites, 9 patients variceal bleeding and four patients other indications for the TIPS. Results: The TIPS was successfully implanted in all patients within 69.6 ± 21.8 min. The shunt reduced the portosystemic pressure gradient by 57.5 ± 14.2% with a mean stent diameter of 7.4 ± 1.0 mm (5 -10.3 mm). During a mean follow-up of 330 ± 249 days, shunt revision was necessary in 5 patients (17%), four of them had insufficient response and received stent dilatation and one patient had stent misplacement requiring a parallel shunt. Three patients (10%) developed HE. Conclusions: The covered, balloon-expandable stent could be placed accurately and allowed creation of adapted shunts with smaller diameters as usual. This resulted in a comparatively low rate of HE.
Keywords: balloon-expandable; stent; portal hypertension; transjugular intrahepatic portosystemic shunt
IntroductionTop
In the beginning of the transjugular intrahepatic portosystemic shunt (TIPS) procedure in the late 1980s and early 1990s balloon-expandable stainless steel stents such as the Palmaz-stent, were used frequently [1-4]. This type of stent allowed stepwise dilatation with the aim to optimize shunt function. Later in the 1990s, numerous brands of nitinol stents were marketed and increasingly used probably because of their better flexibility and easier placement. The release of the polytetrafluoroethylene (PTFE) covered version in 2002 (Viatorr, W.L.Gore, Flagstuff, USA) eventually terminated the implantation of uncovered balloon-expandable metallic stents.
Hepatic encephalopathy (HE) is the major and most frequent complication of the TIPS [5]. Its incidence and severity depend on the individual risk of the patient (pre-TIPS HE, age, Child-Pugh class) and the diameter of the stent which determines the degree of shunting [5-11]. Thus, larger stent diameters with a greater reduction in the portosystemic pressure gradient (the difference between the portal and right atrial pressures) have a higher incidence of TIPS-induced HE. As demonstrated previously, HE occurred almost exclusively in patients with post-TIPS pressure gradients of less than 12 mm Hg, while variceal rebleeding occurred when gradients exceeded the threshold of 12 mm Hg [12, 13]. This suggests a very narrow therapeutic window of the TIPS approaching the threshold of 12 mm Hg. The accurate adjustment of the threshold requires stents with variable diameters. This goal can seldom be achieved by nitinol stents which always expand towards their nominal diameter and which cannot retain a given smaller diameter [14-17]. This is why underdilatation of nitinol stents has an only temporary benefit and does not sufficiently decrease the risk of shunt-induced HE [17].
This study evaluates the usefulness of a balloon-expandable stainless steel stent covered with PTFE. The stent can be dilated to any size between 5 and 12 mm allowing a graded and permanent adjustment of the portosystemic pressure gradient to a desired value.
Patients and methodsTop
The prospective cohort study includes 30 unselected patients who received a TIPS implantation in two centers. Data were deposed in our TIPS-registry which was approved by our local Ethics Commettee. All patients gave their written consent to the recording, analysis and publishing of the data.
Before the TIPS intervention, echocardiography was performed showing an ejection fraction of > 50% and a normal E/A ratio excluding diastolic dysfunction. The TIPS implantation was performed as described previously [4, 18]. Before the intervention, the portal bifurcation was located sonographically and a metallic marker was placed on the skin to approximately indicate its position. After transjugular access, a right portal branch was punctured under sonographic guidance. Splenoportography was then performed and varices occluded with bucrylate when necessary. After measurement of the portosystemic pressure gradient, the parenchymal tract was opacified to exclude arterial or biliary communications. Finally, a covered balloon-expandable stent (Bentley, Hechingen, Germany) was implanted. Stents were dilated gradually and pressure measurements were performed after each dilatation. In patients with HE before the intervention or a bilirubin concentration of above 3 mg/dl, stent expansion aimed at a pressure gradient of 12 ± 1 mmHg. In patients with a baseline pressure gradient of < 20 mmHg pressure gradients were reduced to below 12 mmHg aiming at a reduction by 40 to 50% of baseline. Pressure measurements before and after stent placement were performed in the portal vein and the right atrium to calculate the porto-atrial pressure gradient [19]. Stent diameters were determined from angiographic pictures [20] using the software of the IMPACS system (AGFA IMPAX EE R20 XV SU3, Agfa HealthCare NV - Mortsel, Belgium).
Patients were seen in 3-month intervals after their discharge. A reduction of the post-TIPS portal vein flow velocity by > 50% or to < 30 cm/sec or a stent flow velocity of < 40 cm/sec or > 200 cm/sec were defined as significant shunt insufficiency [18]. In addition, insufficient response or recurrence of the complication(s) of portal hypertension, which indicated the TIPS intervention, gave rise for radiological revision. Clinically overt HE was assessed according to the New-Haven criteria [21].
Statistics
Continuous variables are expressed as mean with standard deviation as well as median with the corresponding range whereas categorial variables are reported as frequencies and percentages. P values of 0.05 or lower were considered statistically significant.
ResultsTop
The characteristics of the patients are summarized in Table 1. Most of the patients had alcoholic cirrhosis of Child-Pugh stage B and received the TIPS intervention for treatment of refractory ascites. Relevant additional complications such as hepato-renal syndrome, hepatic hydrothorax, and HE were seen in 27, 7, and 10% of patients, respectively.
| Variable | Value | Percentage (%) | |
| Age (years) | |||
| mean ± SD | 56.5 ± 9.5 | ||
| median (range) | 62 (32-84) | ||
| Child-Pugh class (n, %) | |||
| A | 9 | 30 | |
| B | 18 | 60 | |
| C | 3 | 10 | |
| Child-Pugh score | |||
| mean ± SD | 8 ± 2 | ||
| median (range) | 8 (5-11) | ||
| MELD score | |||
| mean ± SD | 12 ± 3 | ||
| median (range) | 12 (6-28) | ||
| TIPS indication (n, %) | |||
| Ascites | 17 | 57 | |
| -with hepatorenal syndrome | 8 | ||
| -with Hydrothorax | 2 | ||
| Variceal bleeding | 9 | 30 | |
| Budd-Chiari-syndrome | 1 | ||
| Portal hypertensive gastropathy | 1 | ||
| Portal vein thrombosis | 1 | ||
| Before abdominal surgery | 1 | ||
| High risk or hepatic Encephalopathy before TIPS (n, %) | 3 | 10 | |
| Mean follow-up, days | 330 ± 249 | ||
| Median follow-up, days (range) | 255 (6 - 1029) | ||
Technical aspects
After having punctured the portal vein, the length of the parenchymal tract and the exact position of the hepatic vein exit and the portal vein entrance were determined by retrograde hand injection of contrast dye into the hepatic vein and the tissue tract. Thereafter, the stent was placed. Because of the small size of the device of 5 F only, predilatation of the tissue tract was not necessary before its introduction. As demonstrated in Figure 1, inflation of the balloon leads to expansion of the stent from both ends. This is why migration during stent expansion did not occur and distal as well as proximal ends could be placed with great accuracy. The metallic marker on the skin helps to orientate and facilitates stent placement. Balloon expansion was stopped when a desired smallest diameter of the stent was reached and pressure measurements were performed (Figure 2). If necessary, dilatation was repeated until the desired gradient has been achieved (Figure 3). As shown in Figures 2 and 3, stent visibility is excellent even in patients with tense ascites.
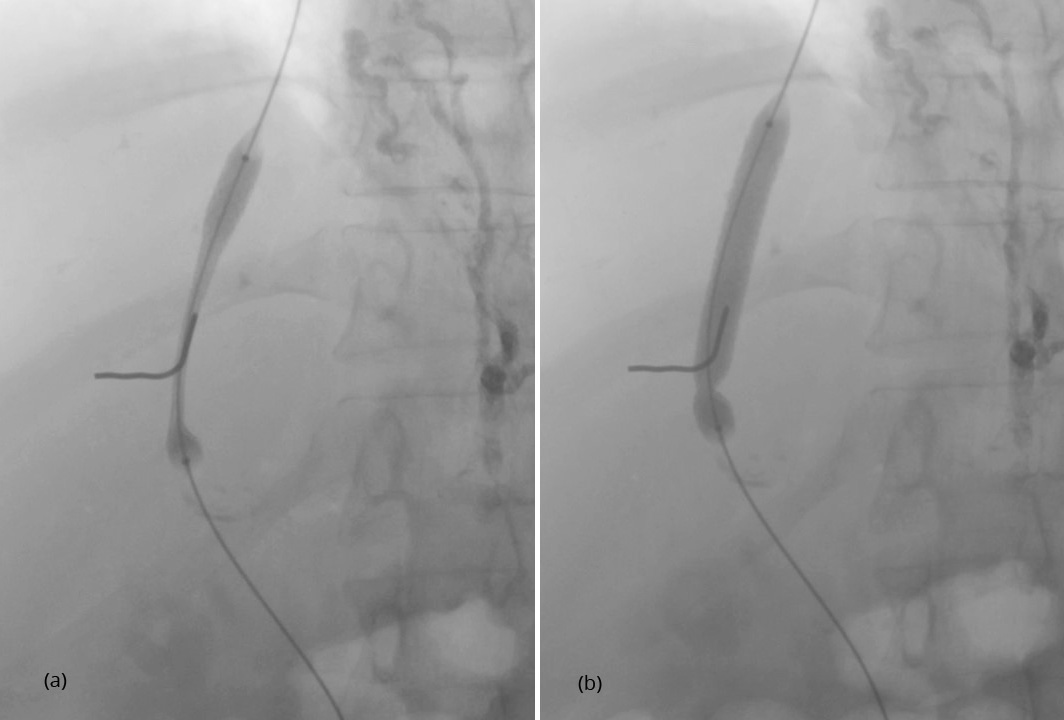
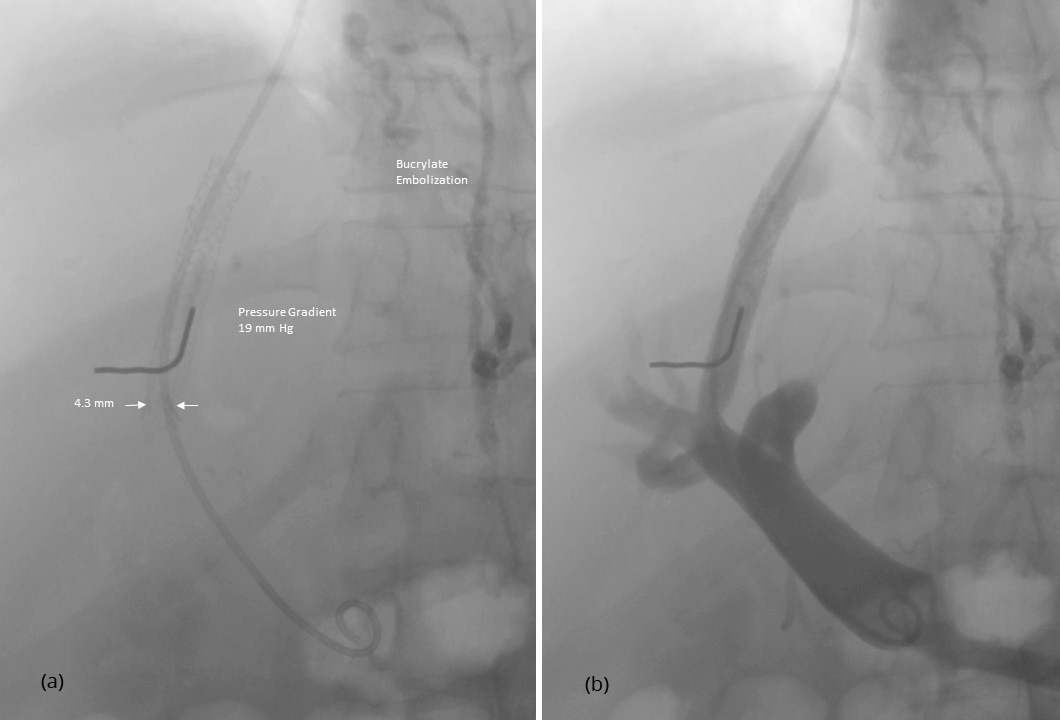
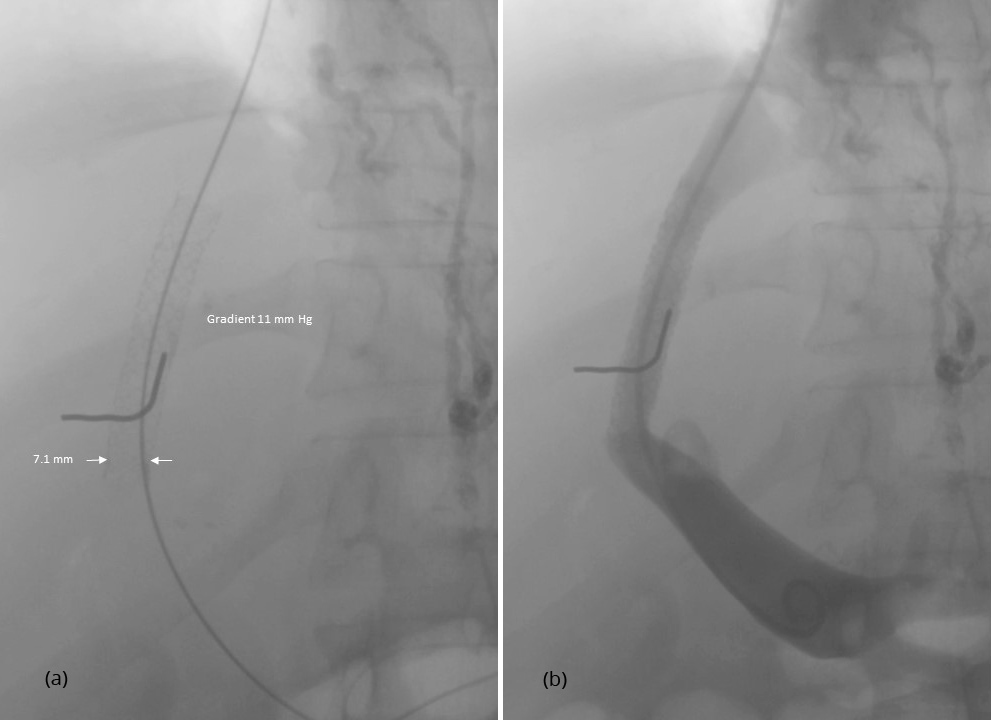
Physical variables of the procedure are summarized in Table 2. The mean pressure gradient after TIPS was 8.9 ± 3.8 mmHg where 21 patients had a final pressure gradient of < 12 mmHg and nine patients a gradient of ≥ 12 mmHg. The smallest diameter of the implanted stent ranged from 5.0 to 10.3 mm with a mean of 7.4 ± 0.95 mm. Twenty patients had a smallest diameter of < 8 mm.
| Number of patients | Mean ± SD | Median (range) | ||
| Pressure gradient before TIPS (mmHg) | 20.5 ± 5.4 | 19 (12 - 38) | ||
| Pressure gradient after TIPS (mmHg) | 8.9 ± 3.8 | 8.0 (1 - 25) | ||
| Number of patients with gradients of | ||||
| < 8 mmHg | 12 | |||
| < 10 mm Hg | 18 | |||
| < 12 mm Hg | 21 | |||
| ≥ 12 mm Hg | 9 | |||
| Reduction of the pressure gradient by TIPS (%) | 57.5 ± 14.2 | 57.6 (30 - 92) | ||
| Smallest stent diameter (radiological measurement, mm) | 7.4 ± 1.0 | 7.6 (5.0 - 10.3) | ||
| Number of patients with a smallest stent diameter of | ||||
| ≤ 6 mm | 4 | |||
| > 6 - ≤ 7 mm | 8 | |||
| > 7 - ≤ 8 mm | 10 | |||
| > 8 mm | 8 | |||
| Duration of intervention (Min) | 69.6 ± 21.8 | 60 (35 - 150) | ||
| Duration of radiation (Min) | 16.4 ± 8.4 | 12 (6 - 38) | ||
Clinical aspects
During follow-up of 330 ± 249 days (median 255, 6-1029 days) rebleeding occurred in 3 (10%) and ascites in 2 patients, all of them received a TIPS revision. HE occurred in 3 patients. One of these patients developed debilitating HE requiring occlusion of the TIPS. Seven patients (23%) died between 137 and 625 days after the TIPS implantation. No patient had clinically overt cardiac decompensation during follow-up after TIPS. This was also true for three patients with elevated pro-BNP concentrations (1021-1139 pg/ml) before TIPSimplantation.
Patency
Patency was assessed by duplex-sonography and confirmed by shunt revision. Five patients without sufficient response received revision. In four of them the patent stent was dilated to further reduce the pressure gradient. Only one patient had shunt insufficiency because the stent did not cover the whole length of the parenchymal tract. A parallel shunt was placed because recatheterization of the shunt was not possible.
DiscussionTop
HE is the most frequent and undesirable adverse event of the TIPS procedure, a reason for exclusion of patients at risk. In spite of this, some patients may develop severe HE after TIPS and require TIPS reduction or occlusion [18, 22]. In addition to patients´ selection, post-TIPS HE may be prevented by reducing the shunt diameter to a degree just needed to effectively improve the indicating symptom. For instance, similar to drug treatment, a 20% reduction in the pressure gradient, which may be reached with small stent diameters of 5 to 7 mm, may be sufficient to prevent variceal rebleeding and to improve survival [23]. This is supported by recent studies and a recent recommendation telling that a reduction of the pressure gradient by 10% effectively prevents rebleeding [24]. In patients with ascites the situation is not as clear. Therefore, wider shunts are implanted to guarantee treatment response.
To reduce the risk of HE, many interventionalists dilated the stents only partially. Secondary enlargement of the diameter was intended in case of insufficient response. However, recent studies demonstrate that nitinol stents always expand until they reach their nominal diameter [14, 17]. Thus, 10 mm stents expanded during follow-up by 1.5 mm almost always reaching their nominal diameter [14]. This is why underdilatation of nitinol stents is of transient value and does not prevent HE [17]. To overcome this problem, a new type of nitinol stent (Viatorr CX) was released in 2016. This stent allows gradual expansion to a diameter of 8, 9 or 10 mm. However, considering the individual risk of HE, smaller shunts (< 8 mm) may be considered in many patients at risk for post-TIPS HE or liver failure. This may be achieved using a balloon-expandable metallic stents which maintains its given diameter over time. In case of insufficient response and lack of side effects, the shunt can be revised and expanded.
This study demonstrates the usefulness of a balloon-expandable, covered stent for creation of the TIPS. Exact placement could be achieved in 29/30 patients and long-term patency was comparable to covered nitinol stents [25, 26]. Most important, stent diameters could be adjusted as warranted to a diameter of 5.0 to 10.3 mm to result in a desired reduction of the pressure gradient. Thus, 20 patients received a stent diameter of < 8 mm and nine patients had a final pressure gradient of ≥ 12 mmHg. This may be the reason for the low incidence of post-TIPS HE of 10% only. In comparison, two recent studies using 8 or 10 mm covered nitinol stents had a risk of HE of 18% and 40%, respectively [27, 28]. On the other hand, non-response may be increased but could easily be resolved by enlargement of the stent.
The placement of the stent is easy and fast. Due to the small diameter of the stent device, predilatation of the tissue tract and introduction of a sheath is not necessary. The stent which is mounted on a balloon catheter can be placed right away. When compared with the covered nitinol stent (Viatorr), the balloon expandable stent is less expensive and the sheath and balloon catheter can be saved as well.
ConclusionTop
This covered and balloon-expandable, metallic stent seems to be ideal in patients with decompensated liver function and a higher risk of hepatic encephalopathy. In these patients, a stent diameter of < 8 mm may reduce severe post-TIPS complications.
Conflicts of interest
Authors declare no conflicts of interest.
ReferencesTop
[1]Palmaz JC. The experimental basis of TIPS: Lessons from the laboratory with the Palmaz Stent. In: Conn HO, Palmaz J, Rösch J, Rössle M, editors. Transjugular intrahepatic portosystemic shunts. New York, Tokyo. Igaku-Shoin Medical Publisher. 1996; 177–196.
[2]Rössle M, Richter GM, Nöldge G, Palmaz JC, Wenz W, et al. New non-operative treatment for variceal haemorrhage. Lancet 1989; 2 (8655):153.Article Pubmed
[3]Richter GM, Palmaz JC, Nöldge G, Rössle M, Siegerstetter V, et al. Der transjugulareintrahepatischeportosystemische Stent-Shunt (TIPSS). Eineneuenichtoperative, perkutaneMethode. Radiologe. 1989: 29:406–411.
[4]Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994; 330(3):165–171.Article Pubmed
[5]Bettinger D, Schultheiss M, Boettler T, Muljono M, Thimme R, et al. Procedure and shunt-related complications and mortality of the transjugular intrahepatic portosystemic shunt (TIPSS). Aliment PharmacolTher. 2016; 44(10):1051–1061.Article Pubmed
[6]Nolte W, Wiltfang G, Schindler C, Münke H, Unterberg K, et al. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: Clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology. 1998; 28(5):1215–1225.Article Pubmed
[7]Rössle M, Piotraschke J. Transjugular intrahepatic portosystemic shunt and hepatic encephalopathy. Dig Dis. 1996; 14 Suppl 1:12–19.Pubmed
[8]Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008; 103(11):2738–2746.Article Pubmed
[9]Jalan R, Elton RA, Redhead DN, Finlayson ND, Hayes PC. Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent-shunt for variceal haemorrhage. J Hepatol. 1995; 23(2):123–128.Article Pubmed
[10]Bai M, Qi X, Yang Z, Yin Z, Nie Y, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: A systematic review. J GastroenterolHepatol. 2011; 26(6):943–951.Article Pubmed
[11]Casadaban LC, Parvinian A, Minocha J, Lakhoo J, Grant CW, et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: Incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015; 60:1059–1066.Article Pubmed
[12]Casado M, Bosch J, Garcia-Pagan JC, Bru C, Bañares R, et al. Clinical events after transjugular intrahepatic portosystemic shunt: Correlation with hemodynamic findings. Gastroenterology. 1998; 114(6):1296–1303.Article Pubmed
[13]Rössle M, Siegerstetter V, Olschewski M, Ochs A, Berger E, et al. How much reduction in portal pressure is necessary to prevent variceal rebleeding? A longitudinal study in 225 patients with a transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2001; 96(12):3379–3383.Article Pubmed
[14]MollaiyanMollaiyan A, Bettinger D, Rössle M. The underdilation of nitinol stents at TIPS implantation: Solution or illusion?Eur J Radiol. 2017; 89:123–128.Article Pubmed
[15]Borghol S, Perarnau JM, Pucheux J, D´Alteroche I, Ayoub J, et al. Short- and long-term evolution of the endoluminal diameter of underdilated stents in transjugular intrahepatic portosystemic shunt. DiagnInterv Imaging. 2016; 97(11):1103–1107.Article Pubmed
[16]Pieper CC, Sprinkart AM, Nadal J, Hippe V, Meyer C, et al. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J VascIntervRadiol. 2015; 26(3):388–394.Article Pubmed
[17]Gaba RC, Parvinian A, Minocha J, Casadaban LC, Knuttinen MG, et al. Should transjugular intrahepatic portosystemic shunt stent be underdilated? J VascIntervRadiol. 2015; 26(3):382–387.Article Pubmed
[18]Rössle M. TIPS: 25 years later. J Hepatol. 2013; 59(5):1081–1093.Article Pubmed
[19]Rössle M, Blanke P, Fritz B, Schultheiss M, Bettinger D. Free hepatic vein pressure is not useful to calculate the portal pressure gradient in cirrhosis: A morphologic and hemodynamic study. J VascIntervRadiol. 2016; 27(8):1130–1137.Article Pubmed
[20]Hoffmann KR, Dmochowski J, Nazareth DP, Miskolczi L, Nemes B, et al. Vessel size measurements in angiograms: Manual measurements. Med Phys. 2003; 30(4):681–688.Article Pubmed
[21]AASLD, EASL. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the European Association for the study of the liver and the American Association for the study of liver diseases. J Hepatol. 2014; 61(3):642–659.Article Pubmed
[22]Schultheiss M, Bettinger D, Boettler T, Thimme R, Rössle M. Severe Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt (TIPS): Value of Shunt Reduction and Occlusion. JSM Hepat. 2017; 2(1):1009.Article
[23]Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rhodes J, et al. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003; 37:902–908.Article Pubmed
[24]De Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015; 63(3):743–752.Article Pubmed
[25]Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: Results of a randomised study. Gastroenterology. 2004; 126(2):469–475.Article Pubmed
[26]Perarnau JM, Le Gouge A, Nicolas C, d´Alteroche L, Borentain P, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: A randomized controlled trial. J Hepatol. 2014; 60(5):962–968.Article Pubmed
[27]Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rössle M, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents v ersus hemodynamically controlled medical therapy. Gastroenterology. 2015; 149(3):660–668.Article Pubmed
[28]Holster L, Tjwa E, Moelker A, Wils A, Hansen BE, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy plus β-blocker for prevention of variceal rebleeding. Hepatology. 2016; 63(2):581–589.Article Pubmed



