Journal of Hepatology and Gastroenterology
An International Peer-Reviewed Open Access Journal
ISSN 2399-8199


- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Hepatology and Gastroenterology
Volume 2, Issue 1, January 2017, Pages 1–6
Original researchOpen Access
Lumbosacral transcutaneous electrical stimulation in children with slow transit constipation: A pilot case series
- 1 Lady Cilento Children’s Hospital, Brisbane, Queensland, Australia
- 2 University of Queensland, Brisbane, Queensland, Australia
*Corresponding author: Harveen Singh, Gastroenterology Department, Lady Cilento Children’s Hospital, Brisbane, Queensland, Australia. Tel.: 3068 1111; Fax: 30683469; E-mail: harveen.singh@health.qld.gov.au
Received 07 October 2016 Revised 19 December 2016 Accepted 25 December 2016 Published 29 December 2016
Copyright: © 2017 Singh H, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Background and aim: This prospective pilot case series evaluated the efficacy of lumbosacral transcutaneous electrical stimulation using interferential current (TESIC) administered with a portable interferential device in children with slow transit constipation (STC). Children with STC are often treatment resistant. TESIC has been successful in some cases. Numerous variations exist for TESIC, with respect to application site, frequency and current settings. This study evaluates an alternative method of TESIC in children with STC to that reported previously. Methods: Eight children with STC failing standard management were treated with TESIC. Treatment was applied to the lumbosacral region twice daily for 30 min for four weeks. Families used small, portable devices at home. TESIC was administered with two electrodes placed in the lower lumbar region and two placed over the gluteal muscles. Patients were monitored for four weeks before and after TESIC therapy. Results: Compared to baseline, there was a significant increase in the number of bowel movements (total and spontaneous) during TESIC therapy (p < 0.05; Wilcoxon signed ranks test). Most patients reported a non-significant improvement in abdominal pain. Similarly, there were non-significant improvements in post TESIC parental and children quality of life scores. Conclusion: This new method of TESIC for STC warrants further evaluation in a randomised, controlled trial.
Keywords: constipation; transcutaneous electrical nerve stimulation; gastrointestinal transit; lumbosacral region
IntroductionTop
Slow transit constipation (STC) is defined by delayed transit on colonic transit studies, using either nuclear or radio-opaque markers [1, 2]. Using nuclear transit studies, STC has been defined as when radioactive tracer reaches the cecum by six hours but most of the tracer is retained in the proximal colon and transverse colon at 24 h, 30 h and 48 h [2], although various criteria have been employed in different centers. STC is emerging as an important cause of chronic constipation in older children and adolescents. 50%-70% of children and adolescents with medication resistant constipation treated in tertiary clinics have STC [2, 3]. Children with STC have irregular bowel movements associated with colicky abdominal pain, uncontrollable soiling and poor quality of life [4].
STC in children is associated with an overall reduction in the frequency of antegrade propulsive contractions in the colon and an absence of the normal increases in colonic motility associated with eating and awaking from sleep [5, 6]. Various factors may contribute, including gastrointestinal neuropathy [7] connective tissue laxity [8].
New therapies for STC are being investigated. Surgical management, with antegrade colonic enemas via an appendicostomy [9, 10] or cecostomy is often effective. In adult patients, colonic resection has been advocated [11]. Enteric nervous system abnormalities have been identified in adults and children with STC [12-14]. Previous studies in adults have demonstrated a reduction in the number of neurones in the colonic myenteric plexus and a reduction in the volume of interstitial cells of Cajal in all layers of the sigmoid colon [12]. Sacral nerve stimulation using implantable devices may improve constipation in adults [15] and children [16-18]. Clearly, non-invasive, transcutaneous therapies are preferable.
Increasingly, transcutaneous electrical stimulation using interferential current (TESIC) is utilised for a variety of conditions, including musculoskeletal pain and urinary incontinence [19]. TESIC has shown promise in the treatment of STC in children and has been shown to produce diarrhea in patients who are being treated for bladder instability [20-26]. It also shows promise in adults with STC [27] and irritable bowel syndrome [28]. Therapy using electrodes applied across the upper abdomen (two electrodes below the costal margin and two paraspinal electrodes between T9 and L2) was effective in children with STC in a small pilot study [15] and subsequent larger trials [22]. In children, receiving daily TESIC, symptoms improved in 50% and colonic transit accelerated in 52% [22]. Quality of life improved as well [23]. 67% of children using TESIC for STC had clinical improvement lasting more than two years in half of them [24].
Colonic manometry has shown that TESIC increases colonic propagating pressure waves, however the precise mechanism is unknown [29]. It has been postulated that electrical impulses from the device may travel across the skin to activate sensory nerve fibres, sensory and motor nerves in spinal nerves, sympathetic and parasympathetic nerves, enteric nerves in the bowel wall or interstitial cells of Cajal [24].
TESIC fires interferential current frequently, in contrast to the traditional TENS (Transcutaneous Electrical Nerve Stimulation) [18]. Interferential current consists of two out of phase alternating currents (AC) with different amplitudes that interfere with each other in the tissue between the two electrodes [18]. The crossing currents in the body are thought to stimulate peripheral nerves [18]. This stimulation may lead to modulation of the extrinsic neural control of the large bowel or modulation of reflexes that inhibit large bowel function [18]. The effect of interferential current can vary depending on the orientation of the nerve fibre to the axis of the current [30]. Other proposed mechanisms of interferential include a decrease in the threshold voltage for sensory nerve excitation when bursts of AC are applied transcutaneously [30]. A single burst may result in multiple action potentials, with the firing frequency being multiples of the burst frequency [30].
The present study is a trial of different electrode positions and stimulation settings of TESIC from that described previously [20-25]. Serendipitously, one of our patients with medication resistant STC discovered an alternative method of electrode placement on the lower back and buttocks. This method of TESIC led to immediate improvement in the child’s constipation symptoms and over time led to long-term remission of her STC. Currently, the use of TESIC in children is limited to small studies of abdominal pad placement and outcomes are variable. The efficacy of this new method of TESIC was assessed in a prospective open labelled pilot study to determine whether alternative pad placement can impact outcomes in STC.
Primary outcomes were total and spontaneous frequency of defecation and quality of life. Secondary outcomes were days with abdominal pain and laxative use. The study was approved by the Royal Children’s Hospital and University of Queensland Ethics committee, (HREC 00175). Written informed consent was obtained from both parents and children participating in the study.
Materials and methodsTop
Children aged 8-17 years with STC diagnosed by nuclear colonic transit study and failing standard management were eligible to participate. Eight children (three males and five females) were enrolled. STC was diagnosed by nuclear colonic transit study as reported by a nuclear medicine physician. Previous studies of TESIC for STC in children have used nuclear transit studies to demonstrate initial diagnosis [18-25].
All children had functional constipation according to Rome III criteria with severe symptoms. They were recruited from tertiary pediatric gastroenterology clinics in major pediatric hospitals in the state of Queensland, Australia. All had normal rectal biopsies, MRI, biochemical workup and neurological examination at commencement. Prior investigations, constipation management regimen and comorbidities were detailed in physician assessments. Organic causes of constipation were excluded prior to enrolment. Children with contraindications to TESIC were excluded. These contraindications include poor skin integrity in the treatment area, impaired sensation in the treatment area, malignancy, bleeding, infection, pregnancy, metal in the treatment area and arterial disease.
All children were medication dependent. Appendicostomies were present in two of the children to enable regular bowel washouts. One child withdrew from the study due to unrelated infectious illness. No child had received TESIC previously. If present, fecal impaction was treated prior to TESIC. Baseline measurements prior to the commencement of the treatment included: bowel dysfunction assessment form (filled out by gastroenterologist, family and physiotherapist), PedsQL questionnaires and weekly bowel diaries.
TESIC was added to treatment as usual, including regular laxatives and behavioural toileting management. TESIC was applied to the lumbosacral region twice daily for 30 min for four weeks. Families used a portable TESIC device suitable for home use. Bowel diaries were recorded continuously from one month prior to TESIC, to one month after completion. In these diaries, patients recorded daily episodes of defecation (during timed toilet visits and spontaneous, urge-initiated visits), fecal incontinence, abdominal pain and medication use (oral, rectal and via appendicostomy if applicable). Bowel movements were described using the Bristol stool scale. Quality of life was recorded at baseline, during TESIC and at the end of the study using the Pediatric Quality of Life Inventory Version 4.0 (both child and parent version).
Stimulation regime
A 9V battery operated TESIC machine (EPM IF 4260, Fuji Dynamics, Hong Kong) was used. Four self-adhesive electrode pads (4 × 4 cm) were used to deliver sinusoidal current. Two electrodes were placed either side of the spine over the paraspinal muscles level with the iliac crest (L4-5). Two other electrodes were placed midway between the ischial tuberosity and greater trochanter on each side. The crossover point was the S2-3 region of the sacrum.
Electrical current was applied twice daily (morning and night) for 30 min, delivered at a 4 KHz frequency. The level of stimulation was standardised for each patient, with a beat frequency of 80-150 Hz, as detailed in previous pediatric TESIC STC studies [18-25]. The intensity level was adjusted to just below sensory threshold and so that visible muscle contractions were not occurring [25].
Each 30 min session consisted of 15 min P2 (a sweep of 2-10 Hz with a sweep cycle 12 sec) and 15 min P4 (set value of 25 Hz.) This time period was chosen to occur at meal times and to conform with the one hour per day regimen used in original pediatric TESIC trials. The aforementioned electrical parameters were adopted from previous studies for bladder stimulation [29]. Correct use of the machine was assisted by physiotherapist education of the patients. Each patient was provided with an instruction sheet. A phone call after two weeks was made by the physiotherapists to ensure adherence.
Patient groups
Ages ranged from 10 to 14 years, (median 12). Height ranged from 1.42 to 1.64 m, (median 1.52 m). Weight ranged from 37 kg to 61 kg, (median 48.5 kg). The patients had symptoms of constipation for between four to nine years. All had been treatment resistant despite specialist pediatric gastroenterology care for two to six years.
TESIC was added to treatment as usual. Patients were kept on the same regimen (medication and dosage) as they were before study initiation. Treatment regimens varied widely. Four patients used oral macrogol, two as monotherapy and two in combination with other agents, one with sodium picosulfate and one with paraffin oil and senna. Two patients used bisacodyl in combination with other agents, one with senna and one with paraffin oil. Two patients used appendicostomy washouts, one with glycerine and saline and the other with glycoprep, epsom salts and sodium picosulfate. Families were encouraged to continue with their timed toileting practice, following main meals in all treatment phases.
In regard to surgical history, two patients had a functioning Malone antegrade continence enema (MACE) stoma. Another patient’s appendicostomy had been removed previously due to infection. An additional patient had a history of lateral sphincterotomy and anal sphincter botulinum toxin injection.
Three of the patients had co-existing bladder symptoms. One patient was allergic to soy and another was lactose intolerant. Three patients had a concomitant diagnosis of autistic spectrum disorder. Other comorbidities included eosinophilic esophagitis, asthma, eczema and congenital hypothyroidism, on adequate replacement therapy. Two of the patients had no comorbidities. Patients were de-identified during analysis.
ResultsTop
Data was recorded as the number of bowel movements (spontaneous bowel movements and timed toileting practice), episodes of abdominal pain and laxative use per day. This data was interpreted in month long periods (pre TESIC, TESIC and post TESIC). Quality of life scores were recorded on one day each month. Wilcoxon Signed Rank test was used to compare the pre TESIC, TESIC and post TESIC month results. Results were evaluated using summary statistics of median and interquartile range. A p value below 0.05 was considered statistically significant.
Spontaneous bowel movements
A significant increase was observed in spontaneous urge-initiated bowel movements when comparing baseline (median 9, IQR 0-34, p 0.049) and TESIC (median15, IQR 2-41). However, the median frequency of bowel movements in the month following TESIC (median 6.5, IQR 0-36.8, p 0.465) was not significantly different to baseline. Only one patient sustained increased bowel movement frequency when compared to baseline as demonstrated in Figure 1. Post treatment data is limited as two participants did not submit their bowel diaries for the post TESIC month, however anecdotally one of these cases reported complete remission of constipation symptoms and did not require further care. The other had multiple significant medical complaints, unrelated to the treatment and had returned to a regional centre after TESIC.
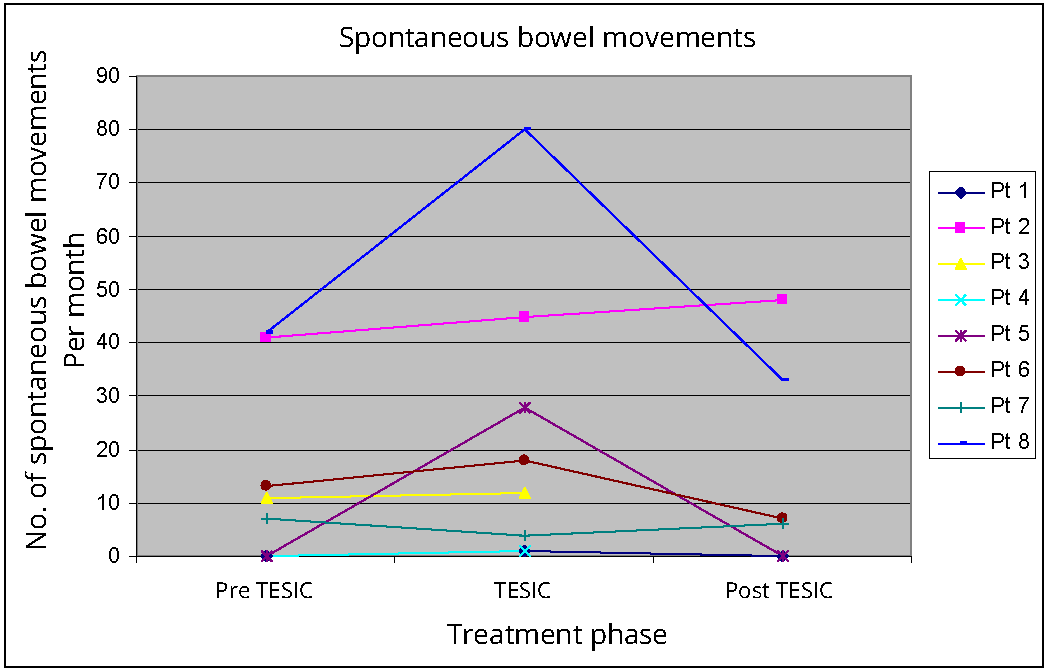
Total number of bowel movements
Total bowel movement frequency increased, when comparing baseline (median 14, IQR 8-37.8, p value 0.049) to TESIC (median 23, IQR 6-48). Post TESIC (median 18.5, IQR 8-37.8, p 0.345) there was no significant improvement in bowel movement frequency when compared to baseline. Two patients were observed to experience a higher frequency of bowel movements, post TESIC when compared to baseline (Figure 2).
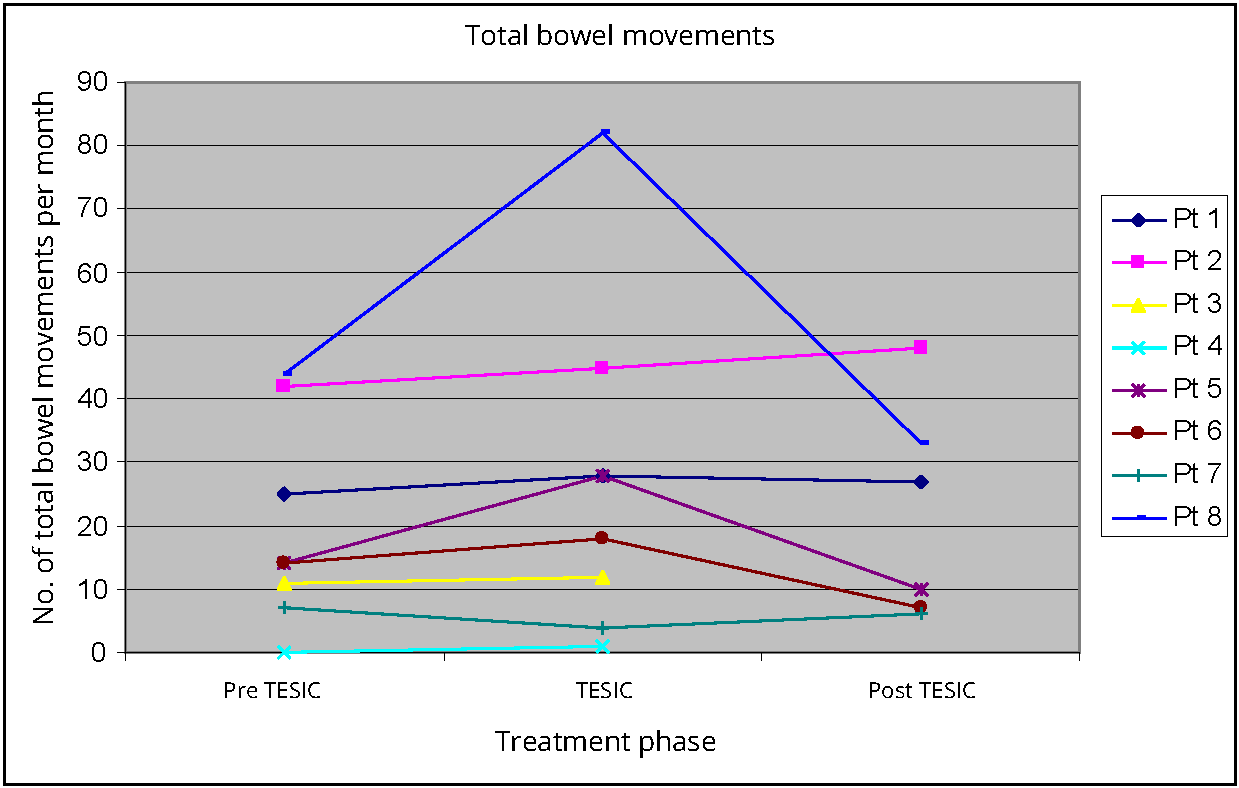
Pediatric quality of life
PedsQL is a validated measure of quality of life in children and their parents [23]. Total QOL scores pre TESIC (median 81, IQR 52.5-85.3, p 0.612) did not significantly improve with TESIC (median 83.7, IQR 47.8-9.1). Post TESIC QOL (median 88.6, IQR 45.9-92.1, p 0.075) also did not show significant improvements when compared to baseline. Median psychosocial QOL pre TESIC (median 82.5, IQR 47.9-84.6, p 0.833) declined when compared to TESIC (median 80.7, IQR 45.8 - 93.7). There was non-significant improvement when comparing post TESIC (median 85.8, IQR 50.4 - 98.7, p 0.075) to pre TESIC scores. Physical QOL demonstrated a non-significant rise from baseline (median 76.6, IQR 61 - 89.1, p 1) when compared to TESIC (median 84.4, IQR 51.6 - 93). After TESIC, physical QOL continued to rise although this did not reach statistical significance (median 96, IQR 37.5 - 100, p 0.715) when compared with baseline.
Parent reported quality of life
Non-significant rises were seen in all domains of parent-reported QOL, both during and after treatment. Parent reported total QOL at baseline was (median 73.9, IQR 42.8 - 80.2, p 0.15), compared to TESIC (median 82.6, IQR 45.9 - 88). Total QOL post TESIC (median 86.4, IQR 25-95, p 0.116) was similar when compared to pre TESIC. Physical QOL at baseline was (median 82.8 IQR 57-89.8, p 0.236) compared to TESIC (median 85.9 IQR 57.8 - 99.5). Post TESIC (median 92.2, IQR 36.7 - 95.3, p 0.596) there was a small improvement compared to baseline. Parent reported psychosocial QOL at baseline was (median 71.1, IQR 32.5 - 77.1, p 0.128) compared to TESIC (median 80.0, IQR 39.6 - 88.3). Post TESIC, (median 93, IQR 22.9 - 93.8, p 0.075) a small increase was noted compared to baseline.
Abdominal pain
Abdominal pain was recorded as days with pain per month. There was non-significant reduction in days with abdominal pain when comparing pre TESIC (median 4, IQR 3 - 15.3, p 0.235) to TESIC (median 2.5, IQR 0 - 16.5). There was similar non-significant reduction in days when comparing post TESIC abdominal pain (median 2, IQR 0 - 11.3, p 0.075) to pre TESIC abdominal pain. Three patients had complete resolution of abdominal pain during treatment, which was sustained in the following month (Figure 3).
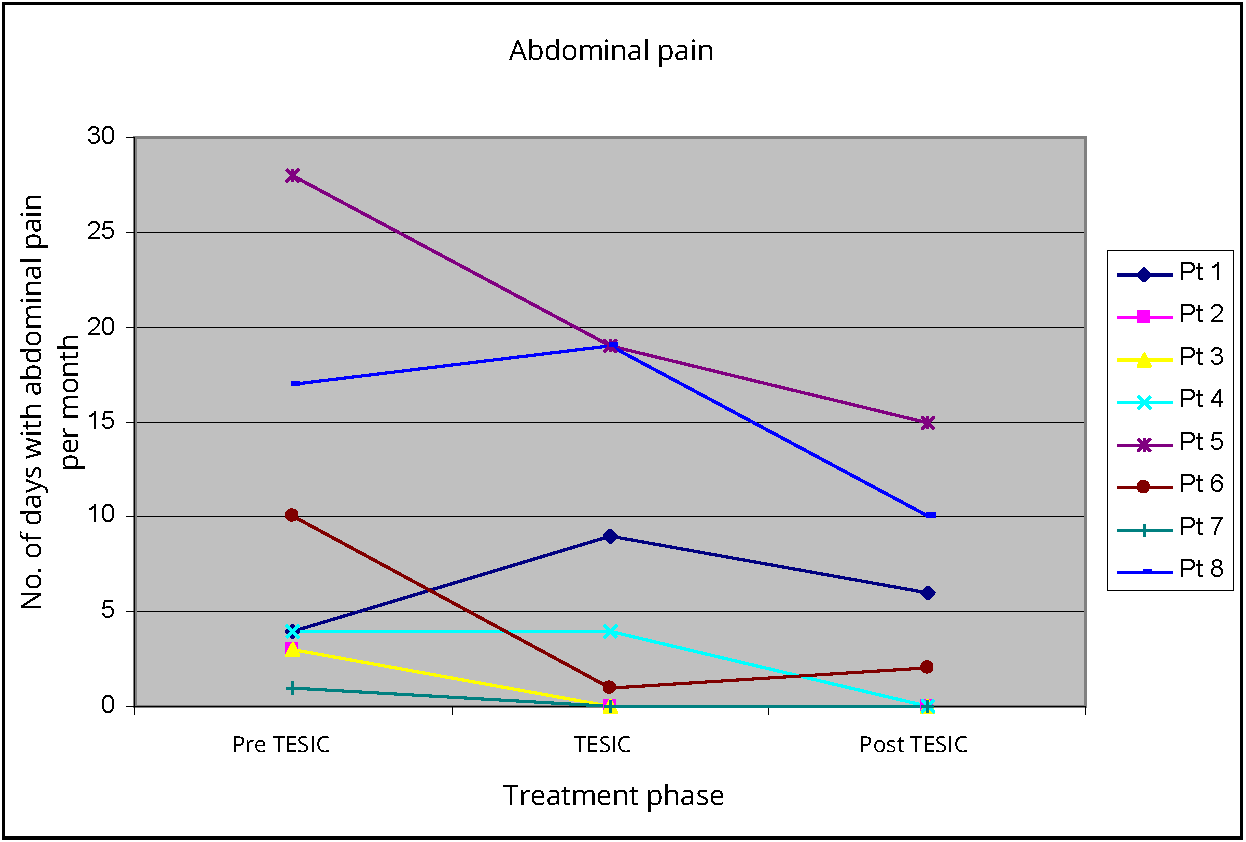
Laxative use
There was a reduction in laxative use from pre TESIC to post TESIC period but this did not reach statistical significance (p 0.72). Similarly, there was decreased frequency of appendicostomy washouts during TESIC treatment, which was sustained following TESIC. As patient’s medication regimens were not standardised, they could not be compared statistically. Change in medication use was calculated as a percentage change from each patient’s baseline dose (grams of macrogol, bisacodyl, senna) or wash out frequency, prior to TESIC treatment. All patients were considered as being on a 100% of their medication dose in the baseline observation period, prior to TESIC.
During the TESIC month four out of the eight patients decreased their medication use/washout frequency in comparison to the pre TESIC month. Two of these patients did not provide their post treatment data. Only one of the children with post treatment data remained on reduced medication dosage after TESIC ceased. The other patient reverted back to their pre TESIC medication use once therapy ceased.
The two children with appendicostomies experienced a decrease in the frequency of washouts during TESIC, but washout frequency increased after TESIC was ceased. An increase in some medications following TESIC was observed in three patients. Only one patient experienced no change with respect to medication use (Figure 4).
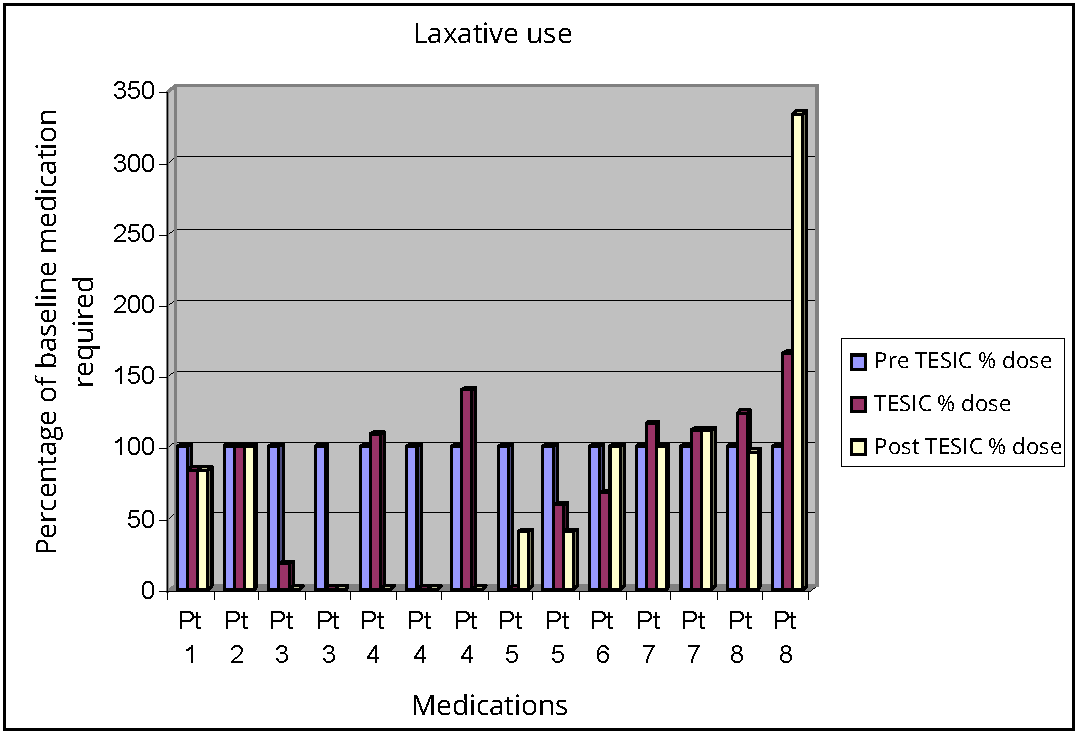
DiscussionTop
This method of lumbosacral TESIC shows promise for improving defecation frequency in children with STC. Despite the small patient numbers, there were significant increases in total and spontaneous bowel movements during TESIC treatment but the effect of TESIC on defecation frequency in the post treatment month did not reach significance. Long-term effects of TESIC in STC have been varied, with one third of patients perceiving an improvement in defecation lasting more than two years and one third lasting less than six months [26]. Further research to the long-term efficacy of TESIC for constipation in children is needed. In the short term, TESIC has led to increased defecation in children with STC. The use of TESIC in children with STC has resulted in significant increases in colonic transit according to nuclear transit studies at 24 h, 30 h and 48 h [20].
Adults with STC who received direct stimulation of sacral nerve S3 have previously demonstrated an increase in pan-colonic propagating sequences [30]. The lumbosacral method aims for interferential current to cross the S3 area, this may lead to sacral nerve stimulation, which has been shown to be useful in the treatment of refractory functional constipation [16-18]. Electrical stimulation via S2-S5 can excite autonomic and somatic nervous system and cause direct and reflex mediated responses in fecal continence mechanisms [18]. It has been suggested that the effect is mediated by afferent sensory nerves which modify the ascending supraspinal control of defecation [31]. Since our case series was conducted, the use of abdominal pad placement in the S2-S4 region has demonstrated effectiveness in children with STC and anorectal retention [31].
Quality of life is a concern for children with STC. PedsQOL scores, although not reaching significance, showed the greatest improvement in pediatric and parental psychosocial QOL. These psychosocial scores cover emotional, social and school functioning. The existing literature on quality of life in children with STC is small. Thus far, significant decreases in QOL in children with STC compared to controls has been demonstrated in an Australian study [4]. In children with STC undergoing TESIC, a significant improvement in physical and psychosocial QOL was experienced when compared to baseline, those who underwent placebo TESIC did not experience such an improvement [23].
QOL is often thought to improve in children who have had procedures such as appendicostomies for STC. The present study was not powered to detect an improvement in quality of life, however trends suggest a benefit may be achieved if the therapy is evaluated in a larger trial in the future. No child reported irritation from pad placements or pain or discomfort from equipment usage.
Abdominal pain can also contribute to poor QOL. No significant improvements in abdominal pain have been found in previous trials using daily TESIC over one or more months [8, 22]. Similarly, we found a non-significant decrease in days with abdominal pain, with three patients becoming free of abdominal pain in the post TESIC period.
There were several limitations to this pilot case series. It was not powered to find statistically significant improvements in many outcome variables. The significant defecation results may have been due to the duration of therapy rather than pad placement, as one hour per day of TESIC for four weeks has been shown previously to be effective in increasing defecation [21]. Factors that could have affected bowel movements such as changes in diet or lifestyle were not controlled, although patients were advised to not change their diet or toileting habit.
In addition to sample size limitations, data was incomplete for the post treatment follow up phase. The significance of the increase in frequency of bowel movements post TESIC was affected by the failure of two patients to return post treatment bowel diaries. Also, the patient group was heterogeneous with respect to comorbidities, laxatives and surgical history. Only one patient had a documented decrease in laxative use at the end of the post TESIC month. Furthermore, the diagnosis of slow transit in this study was made by nuclear medicine physicians, rather than by specific metrics on nuclear transit study. Of note, TESIC has been shown to be effective in children with both STC and outlet type constipation [31]. The follow up period was brief.
This new method of TESIC for STC warrants further evaluation and can provide the basis for a power calculation to design a larger trial. A larger randomised control trial with clear inclusion criteria and standardised laxative therapy is planned to compare the efficacy of the lumbosacral method versus the traditional trans-abdominal application pioneered by the Melbourne group. Future studies should concentrate on accurate patient classification, by Rome criteria as well as by defined criteria on nuclear transit studies. Abdominal pain severity should be monitored by visual analogue scale in addition to pain frequency by diary recordings. Anticipated benefits include improved frequency of bowel movements, decreased days with abdominal pain and reduced laxative use. Follow up should be for a minimum of twelve months to evaluate the persistence of any beneficial effects.
In the future, use of portable TESIC machines may prevent more invasive measures such as surgery for children suffering from STC.
ConclusionTop
TESIC represents a simple, outpatient treatment that could be monitored by a physiotherapist, potentially reducing health care costs in children with STC.
Funding and conflicts of interest
The authors received funding from the Royal Children’s Hospital allied health grant, however the authors have no links and affiliations, nor do they have any potential conflicts of interest.
ReferencesTop
[1]Constipation in children and young people: Diagnosis and management of idiopathic childhood constipation in primary and secondary care: NICE Clinical Guidelines, No 99. National Collaborating Centre for Women and Children's Health (UK). London: RCOG Press; 2010.Article Pubmed
[2]Cook BJ, Lim E, Cook D, Hughes J, Chow CW, et al. Radionuclear transit to assess sites of delay in large bowel transit in children with chronic idiopathic constipation. J Pediatr Surg. 2005; 40(3):478–483.Article Pubmed
[3]Zaslavsky C, De Barros SG, Gruber AC, MacIel AC, Da Silveira TR. Chronic functional constipation in adolescents: Clinical findings and motility studies. J Adolesc Health. 2004; 34(6):517–522.Article Pubmed
[4]Clarke MC, Chow CS, Chase JW, Gibb S, Hutson JM, et al. Quality of life in children with slow transit constipation. J Pediatr Surg. 2008; 43(2):320–324.Article Pubmed
[5]Stanton MP, Hutson JM, Simpson D, Oliver MR, Southwell BR, et al. Colonic manometry via appendicostomy shows reduced frequency, amplitude, and length of propagating sequences in children with slow-transit constipation. J Pediatr Surg. 2005; 40(7):1138–1145.Article Pubmed
[6]King SK, Catto-Smith AG, Stanton MP, Sutcliffe JR, Simpson D, et al. 24-Hour colonic manometry in pediatric slow transit constipation shows significant reductions in antegrade propagation. Am J Gastroenterol. 2008; 103(8):2083–2091.Article Pubmed
[7]Bassotti G, Villanacci V. Slow transit constipation: A functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006; 12(29):4609–4613.Article Pubmed
[8]Reilly DJ, Chase JW, Hutson JM, Clarke MC, Gibb S, et al. Connective tissue disorder--a new subgroup of boys with slow transit constipation J Pediatr Surg. 2008; 43(6):1111–1114.Article Pubmed
[9]Marshall J, Hutson JM, Anticich N, Stanton MP. Antegrade continence enemas in the treatment of slow-transit constipation. J Pediatr Surg. 2001; 36(8):1227–1230.Article Pubmed
[10]King SK, Sutcliffe JR, Southwell BR, Chait PG, Hutson JM. The antegrade continence enema successfully treats idiopathic slow-transit constipation. J Pediatr Surg. 2005; 40(12):1935–1940.Article Pubmed
[11]Wong SW, Lubowski DZ. Slow-transit constipation: Evaluation and treatment. ANZ J Surg. 2007; 77(5):320–328.Article Pubmed
[12]El-Salhy M. Chronic idiopathic slow transit constipation: Pathophysiology and management. Colorectal Dis. 2003; 5(4):288–296.Pubmed
[13]Tomita R. Regulation of the peptidergic nerves (substance P and vasoactive intestinal peptide) in the colon of women patients with slow transit constipation: An in vitro study. Hepatogastroenterology. 2008; 55(82-83):500–507.Pubmed
[14]Southwell BR, King SK, Hutson JM. Chronic constipation in children: Organic disorders are a major cause. J Paediatr Child Health. 2005; 41(1-2):1–15.Article Pubmed
[15]Thomas GP, Dudding TC, Rahbour G, Nicholls RJ, Vaizey CJ. Sacral nerve stimulation for constipation. Br J Surg. 2013; 100(2):174–181.Article Pubmed
[16]Humphreys MR, Vandersteen DR, Slezak JM, Hollatz P, Smith CA, et al. Preliminary results of sacral neuromodulation in 23 children. J Urol. 2006; 176(5):2227–2231.Article Pubmed
[17]Roth TJ, Vandersteen DR, Hollatz P, Inman BA, Reinberg YE. Sacral neuromodulation for the dysfunctional elimination syndrome: a single center experience with 20 children. J Urol. 2008; 180(1):306–311.Article Pubmed
[18]van Wunnik BP, Peeters B, Govaert B, Nieman FH, Benninga MA, et al. Sacral neuromodulation therapy: A promising treatment for adolescents with refractory functional constipation. Dis Colon Rectum. 2012; 55(3):278–285.Article Pubmed
[19]Bower WF, Yeung CK. A review of non-invasive electroneuromodulation as an intervention for non-neurogenic bladder dysfunction in children. Neurourol Urodyn. 2004; 23(1):63–67.Article Pubmed
[20]Clarke MC, Chase JW, Gibb S, Robertson VJ, Catto-Smith A, et al. Decreased colonic transit time after transcutaneous interferential electrical stimulation in children with slow transit constipation. J Pediatr Surg. 2009; 44(2):408–412.Article Pubmed
[21]Chase J, Robertson VJ, Southwell B, Hutson J, Gibb S. Pilot study using transcutaneous electrical stimulation (interferential current) to treat chronic treatment-resistant constipation and soiling in children. J Gastroenterol Hepatol. 2005; 20(7):1054–1061.Article Pubmed
[22]Yik YI, Ismail KA, Hutson JM, Southwell BR. Home transcutaneous electrical stimulation to treat children with slow-transit constipation. J Pediatr Surg. 2012; 47(6):1285–1290.Article Pubmed
[23]Clarke MC, Chase JW, Gibb S, Hutson JM, Southwell BR. Improvement of quality of life in children with slow transit constipation after treatment with transcutaneous electrical stimulation. J Pediatr Surg. 2009; 44(6):1268–1272.Article Pubmed
[24]Leong LC, Yik YI, Catto-Smith AG, Robertson VJ, Hutson JM, et al. Long–term effects of transabdominal electrical stimulation in treating children with slow-transit constipation. J Pediatr Surg. 2011; 46(12):2309–2312.Article Pubmed
[25]Ismail KA, Chase J, Gibb S, Clarke M, Catto-Smith AG, et al. Daily transabdominal electrical stimulation at home increased defecation in children with slow-transit constipation: A pilot study. J Pediatr Surg. 2009; 44(12):2388–2392.Article Pubmed
[26]Kajbafzadeh AM, Sharifi-Rad L, Nejat F, Kajbafzadeh M, Talaei HR. Transcutaneous interferential electrical stimulation for management of neurogenic bowel dysfunction in children with myelomeningocele. Int J Colorectal Dis. 2012; 27(4):453–458.Article Pubmed
[27]Queralto M, Vitton V, Bouvier M, Abysique A, Portier G. Interferential therapy: A new treatment for slow transit constipation. A pilot study in adults. Colorectal Dis. 2013; 15:e35–39.Article Pubmed
[28]Coban S, Akbal E, Koklu S, Koklu G, Ulasli MA, et al. Clinical trial: Transcutaneous interferential electrical stimulation in individuals with irritable bowel syndrome - a prospective double-blind randomized study. Digestion. 2012; 86(2):86–93.Article Pubmed
[29]Clarke M, Catto-Smith A, King S, Dinning P, Cook I, et al. Transabdominal electrical stimulation increases colonic propagating pressure waves in pediatric slow transit constipation. J Pediatr Surg. 2012; 47:2279–2284.
[30]Ward AR. Electrical stimulation using kilohertz-frequency alternating current. Phys Ther. 2009; 89(2):181–190.Article Pubmed
[31]Hutson J, Dughetti L, Stathopoulos L, Southwell B. Transabdominal electrical stimulation (TES) for the treatment of slow transit constipation (STC). Paediatr Surg Int. 2015; 31(5):445–451.Article Pubmed



