Journal of Clinical and Interventional Cardiology
An International Peer-Reviewed Open Access Journal
ISSN 2399-8202

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Clinical and Interventional Cardiology
Volume 1, Issue 1, June 2016, Pages 1–6
Original researchOpen Access
Effects of coronary revascularization on T-wave amplitude variability in patients with non ST elevated acute myocardial infarction
- 1 UO Cardiologia – UTIC, A.O. San Paolo, Milan, Italy
- 2 UOC Malattie Cardiovascolari, Fondazione IRCSS Ca’ Granda Ospedale Maggiore Policlinico, Dipartimento di Scienze Cliniche e di Comunità, University of Milan, Milan, Italy
*Corresponding author: Federico Lombardi, UOC Malattie Cardiovascolari, Fondazione IRCSS Ca’ Granda Ospedale Maggiore Policlinico, Dipartimento di Scienze Cliniche e di Comunità, University of Milan, Milan, Italy. Tel/Content/CurrentIssues/17/Jan_0001/2399-8202.2016-1/Fax: +39 02 50320483; E-mail: federico.lombardi@unimi.it
Received 26 February 2016 Revised 11 May 2016 Accepted 21 May 2016 Published 30 May 2016
DOI: http://dx.doi.org/10.14312/2399-8202.2016-1
Copyright: © 2016 Moro E, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Introduction: T-wave amplitude variability (TAV) is a noninvasive index of beat-to-beat variations in ventricular repolarization. Aim of the study was to evaluate whether this parameter might reflect the changes in ventricular repolarization process induced by myocardial reperfusion, in the setting of acute myocardial ischemia. Methods and results: 97 patients with diagnosis of non-ST-elevation myocardial infarction (NSTE-MI) eligible for coronary revascularization were studied. In each patient, a 20 minute three pseudo-orthogonal lead recording was obtained at admission and within few hours from angiography. TAV was computed with SyneTVar 3.10a software (ELA medical, SORIN Group, Paris, France). Twenty patients were excluded for technical reason. The remaining 77 patients, aged 68 ± 12.2 years, had a preserved left ventricular ejection fraction (54 ± 10.9 %). In comparison to the first measurement, there was a significant increase in mean TAV (from 20.4 ± 6.7µV to 23.2 ± 9.4µV, P = 0.02) only in patients who underwent coronary revascularization. The increase was more easily detectable in patients with revascularization of left anterior descending coronary artery (from 19.2 ± 7.4µV to 24.3 ± 10.2µV, P = 0.01). No differences were instead observed in patients who were not revascularized for either the lack of critical coronary stenosis or anatomical reasons. Conclusions: Percutaneous coronary revascularization in NSTE-MI patients is associated with a significant increase in TAV, which is likely to reflect the alterations of cardiac electrical properties induced by myocardial tissue reperfusion.
Keywords: myocardial ischemia; reperfusion arrhythmias; T-wave variability; ventricular repolarization; Holter recording
IntroductionTop
Identification of patients at risk for malignant ventricular arrhythmias is a main clinical challenge [1, 2]. Among noninvasive techniques developed to identify patients at risk, measurements of alterations in ventricular repolarization process have provided promising results [3-6]. Microvolt T-wave alternans (mTWA) has long been considered a sensitive and efficient noninvasive methodology to detect alterations in repolarization process and to predict arrhythmic events [1, 7-9]. Whereas negative predictive value is consistently above 95% [7, 8, 10-12], its positive predictive value remains unsatisfactory [7, 13]. As a result, several high risk patients with normal left ventricular ejection fraction (LVEF) are not appropriately identified, treated and therefore remain exposed to an increased arrhythmic risk. In this context, a recent technique named T-wave amplitude variability (TAV) has been proved effective in identifying arrhythmic risk in heart failure [14, 15], Chagas disease [16], long-QT syndrome [17], dilated cardiomyopathy [18] and in malignant arrhythmia survivors without structural heart disease [19]. Unlike the mTWA, this approach does not look for a repeating pattern in repolarization process, but it quantifies the amount of beat-to-beat changes in ST-segment and T-wave amplitude. This phenomenon can be considered an expression of a non-periodic variability in ventricular recovery, which, conceptually, is likely to be prodromal to T-wave alternans [14].
The aim of this study was to evaluate whether TAV might reflect the changes in ventricular repolarization process induced by acute myocardial reperfusion in the setting of non-ST-elevation acute myocardial infarction (NSTE-MI).
MethodsTop
Patients population
Patients’ recruitment was carried out at Coronary Intensive Care Unit of Azienda Ospedaliera San Paolo, University of Milan, Milan, Italy. We enrolled patients hospitalized over one year for non-ST-elevation acute coronary syndrome (NSTE-ACS) with elevation of cardiac biomarker troponin I, eligible for diagnostic coronary angiography [20, 21]. Exclusion criteria were: (i) atrial fibrillation or flutter, artificial paced ventricular rhythm or any other non-sinusal rhythm; (ii) left bundle branch block; (iii) major significant comorbidity (i.e., acute renal failure, hepatic failure).
Study protocol and general procedures
This investigation was designed as an observational cohort study. The Research Ethics Board of the hospital approved the study protocol. All subjects signed a written informed consent before enrollment. All patient were submitted to the common clinical and instrumental procedures performed in patients admitted for NSTE-MI (chest X-ray, standard 12-lead ECG, blood tests, echocardiogram), received standard pharmacological therapy (double antiplatelet therapy, beta-blockers, angiotensin-converting enzyme inhibitors, statin) consistent to their clinical conditions, and underwent diagnostic coronary angiography within 72hrs from admission, as recommended by current guidelines [21]. For each patient, a 3-lead Holter-ECG recording was recorded at admission and within 24hrs after coronary angiography, during the daytime, in resting condition and in absence of noise.
T-wave amplitude variability
Holter ECG recordings were performed using SpiderView digital Holter recorder (ELA medical, SORIN Group, Paris, France) at a sampling rate of 1000Hz, with a resolution of 2,5 µV, for a period of 20 minutes. Electrodes were placed in a pseudo-orthogonal x, y, z lead configuration. ECG files were converted to standard Holter format defined by International Society for Holter and Noninvasive Electrocardiology [22] and analyzed using the Holter reading software SyneScope 3.10 (ELA medical, SORIN Group, Paris, France). T-wave amplitude variability was evaluated using SyneTVar 3.10a software (ELA medical, SORIN Group, Paris, France) based on the vector magnitude VM = √x2+y2+z2, as previously described [14-19, 23]. In brief, ECG signal was first preprocessed by the software, including a down sampling to 200Hz, an estimation of the baseline and respiratory components as well as a digital filtering removal of recording sections characterized by: (i) noise level >10µV, (ii) ventricular or atrial premature beats, or (iii) high RR interval variability (standard deviation >150ms). Multiple clusters made of 60 QRS-T consecutive cycles were created and every repolarization phase (i.e. ST-segment and T-wave) was subdivided in 8 consecutive 50ms segments (T1-T8) following QRS offset (defined as QRS onset + 120ms). Level of variability is computed basing on the variance of the average amplitude of every T1-T8 segment across the consecutive 60 QRS-T cycles of a given cluster. The square root value (i.e. standard deviation) is reported and expressed in microvolt. T-wave amplitude variability (TAV in µV) was defined as the median standard deviation value obtained from every segment of repolarization among all available clusters.
Statistical analysis
Kolmogorov-Smirnov test was used to verify the normality of distribution. Data concerning continuous variables were expressed as means ± standard deviation, whereas data obtained from non-normally distributed variables as absolute numbers and percentages. For comparisons, we used paired and unpaired Student’s t-test, Wilcoxon matched-pair test, Mann-Whitney U test and exact Fisher test, according to variable type and distribution. Correlations were assessed by Spearman's rank correlation method. For all tests, a P value of <0.05 was considered statistically significant. Data were analyzed using NCSS version 9 (NCSS Statistical Software, Kaysville, Utah, USA).
ResultsTop
Study population characteristics
Holter recordings were collected from 97 patients. Twenty of them were excluded from the study because of extremely noised recordings, mainly due to low- or high-frequency artefacts disturbances or frequent ventricular ectopies, which did not permit TAV analysis. The remaining 77 patients sample consisted predominantly by male (58 patients, 75.3%) with a mean age of 68 ± 12.2 years. Median LVEF was 54 ± 10.9%. All patients received optimal medical therapy, when tolerated: aspirin (76 patients, 98.7%), clopidogrel (65 patients, 84.4%), angiotensin-converting enzyme inhibitors (59 patients, 76.6%), beta-blockers (65 patients, 84.4%) and statin (77 patients, 100%). Four patients (5.2%) were on amiodarone at the time of admission. Other baseline characteristics of the study population including heart rate, QT and QTc interval are shown in Table 1.
Due to the finding of a hemodynamically significant stenosis during coronary angiography, 49 patients (63.6%) underwent percutaneous transluminal coronary angioplasty (PTCA), with intracoronary stenting. The treated infarct-related artery (IRA) was respectively: left anterior descending artery (LAD) in 36.7%, left circumflex artery (CX) in 30.6% and right coronary artery (RC) in 32.7%. The remaining 28 patients did not undergo coronary revascularization procedure either for the absence of a hemodynamically significant stenosis or for an indication for surgical revascularization. Two separate groups of revascularized and non-revascularized subjects were therefore considered. There were no significant differences between the two groups when considering heart rate, QT and QTc interval, systolic arterial pressure, left ventricular ejection fraction and clinical characteristics, except for gender, active smoking and diabetes mellitus (Table 1).
| All subjects (n = 77) |
Revascularized (n = 49) |
Non-Revascularized (n = 28) |
P value | |
| Age (years) | 68.1 ± 12.2 | 67.2 ± 12.7 | 69.8 ± 11.3 | 0.37 |
| Male gender | 58 (75.3) | 42 (85.7) | 16 (57.1) | 0.01 |
| Active smoking | 44 (57.1) | 33 (67.3) | 11 (39.9) | 0.03 |
| Hypertension | 46 (59.7) | 30 (61.2) | 16 (57.1) | 0.81 |
| Hyperlipidemia | 39 (50.6) | 25 (51) | 14 (50) | 1.00 |
| Diabetes mellitus | 23 (29.9) | 19 (38.8) | 4 (14.3) | 0.03 |
| Prior MI | 22 (28.6) | 17 (34.7) | 5 (17.9) | 0.18 |
| LVEF (%) | 54 ± 11 | 56 ± 9 | 51 ± 10 | 0.44 |
| Aspirin | 76 (99) | 48 (98) | 28 (100) | 1.00 |
| Clopidogrel | 65 (84) | 46 (94) | 19 (68) | 0.24 |
| β-blocker | 65 (84) | 42 (86) | 21 (75) | 0.35 |
| ACE-inhibitor | 59 (77) | 48 (98) | 19 (68) | 0.26 |
| Statin | 77 (100) | 49 (100) | 28 (100) | 1.00 |
| Amiodarone | 4 (5.2) | 2 (4.1) | 2 (7.1) | 0.61 |
| Heart rate (bpm) | ||||
| Before CAG | 67 ± 14 | 66 ± 11 | 70 ± 20 | 0.87 |
| After CAG | 68 ± 12 | 67 ± 11 | 69 ± 14 | 0.70 |
| QT (ms) | ||||
| Before CAG | 398 ± 48 | 400 ± 39 | 394 ± 63 | 0.74 |
| After CAG | 399 ± 45 | 402 ± 35 | 394 ± 62 | 0.71 |
| QTc (ms) | ||||
| Before CAG | 415 ± 37 | 415± 36 | 414 ± 40 | 0.62 |
| After CAG | 420 ± 40 | 423 ± 38 | 415 ± 44 | 0.56 |
Abbreviations: MI = myocardial infarction; LVEF = left ventricular ejection fraction; ACE-inhibitors = angiotensin-converting enzyme inhibitor; CAG = coronary angiography; QTc = QT interval correct for heart rate by Bazett’s formula. Data expressed as mean ± SD or absolute numbers (percentage). P value of comparison between revascularized and non-revascularized are presented..
T-wave amplitude variability
At admission, mean TAV measured in the entire study population was 20.5 ± 7.1µV. No significant changes were observed after coronary angiography (mean TAV 23.2 ± 8.8µV, P = 0.84). When the study population was analyzed in term of performing effective revascularization, no significant differences were detectable in the two groups when considering admission recordings before coronary angiography (20.4 ± 6.7 vs 20.6 ± 7.7µV, P = 0.88). On the contrary, TAV significantly increased in revascularized patients (from 20.4 ± 6.7µV to 23.2 ± 9.4µV, P = 0.02): this change was particularly evident in the first five segments (T1-T5) of repolarization, which correspond to ST-segment and ascendant phase of T-wave (Figure 1). No changes were instead detectable when comparing admission and post angiography recordings in non-revascularized patients (from 20.6 ± 7.7 to 23.1 ± 7.7µV, P = 0.17) (Figure 2).
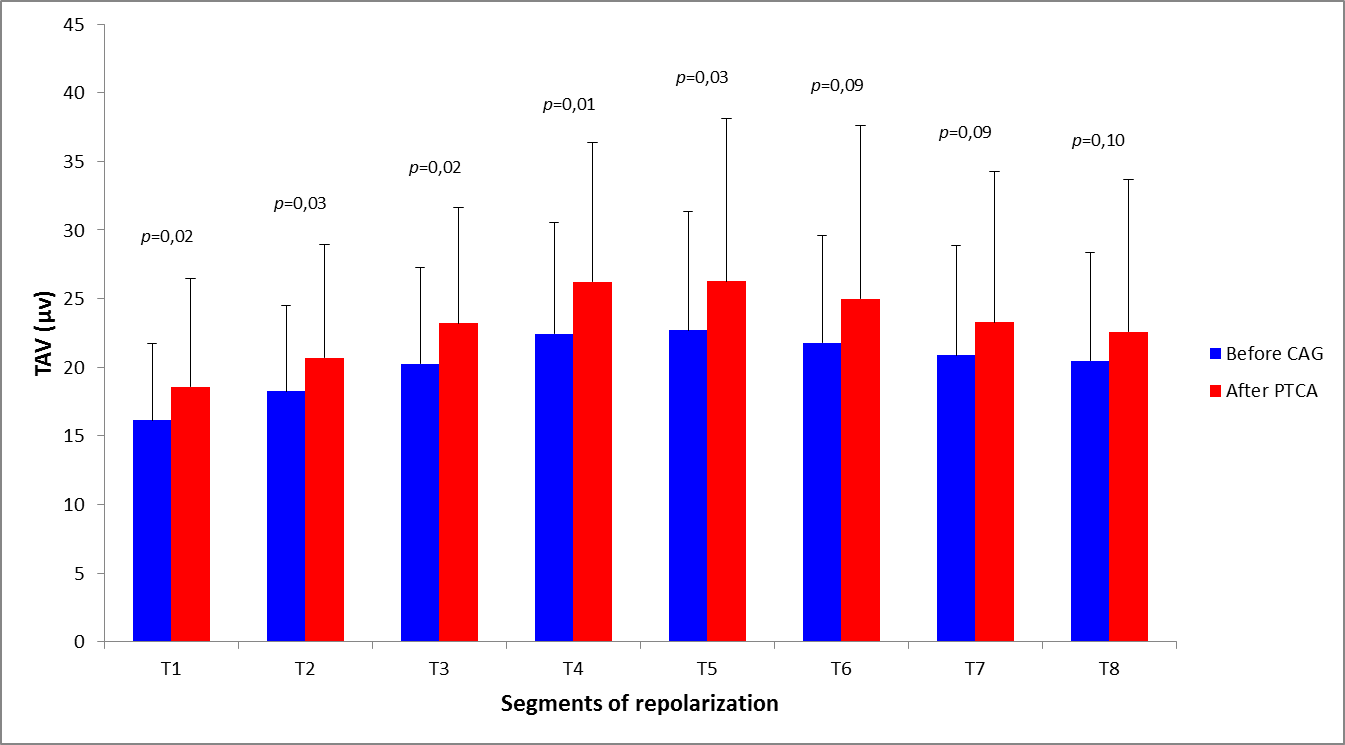
Abbreviations: CAG = coronary angiography; PTCA = percutaneous transluminal coronary angioplasty; TAV = T-wave amplitude variability.
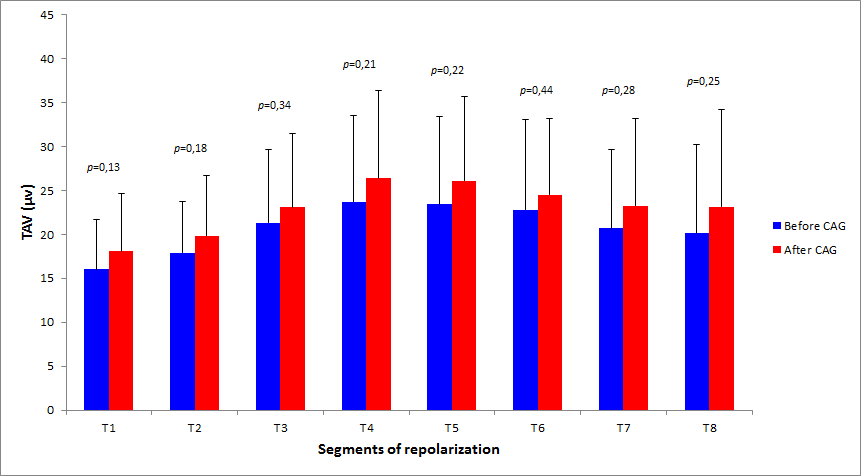
Abbreviations: CAG = coronary angiography; TAV = T-wave amplitude variability
When considering the effects of reperfusion in relation to the treated vessel, we observed a significant increase of TAV in the first five segments of repolarization in patients treated on LAD coronary artery (from 19.2 ± 7.4µV to 24.3 ± 10.2µV, P = 0.01) (Figure 3), whereas no differences were detectable in patients who underwent revascularization of CX or RC artery (from 21.1 ± 6.4 to 22.6 ± 9.1µV, P = 0.34).
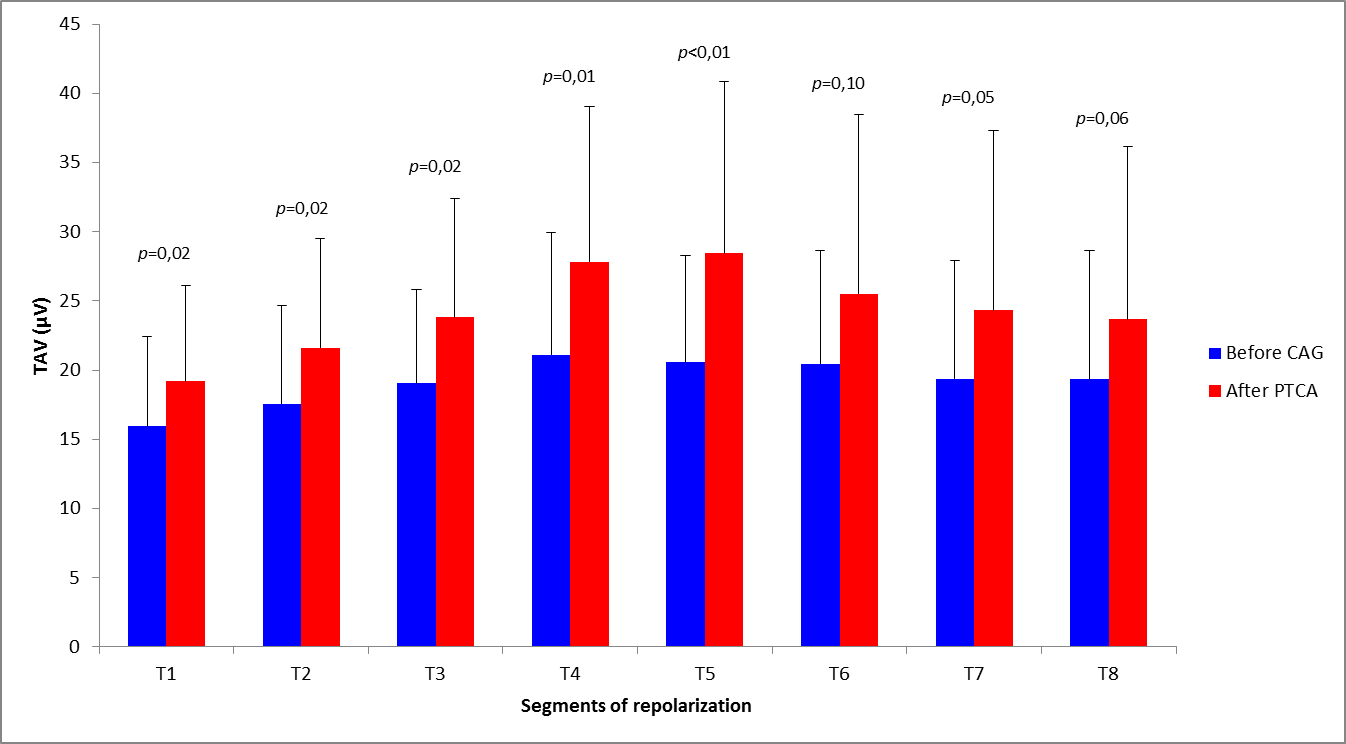
Abbreviations: CAG = coronary angiography; PTCA = percutaneous transluminal coronary angioplasty; TAV = T-wave amplitude variability
In patients subjected to LAD revascularization, the greatest increase in TAV was observed within T4 and T5 segments, which correspond to the ascendant-descendant phase of T-wave (i.e. 320-370ms after QRS onset).
In patients who were not treated with PTCA, there were no differences between patients with or without three vessel disease when comparing TAV measured at admission and after coronary angiography (from 21.8 ± 5.9 to 25.4 ± 8.3µV, P = 0.15; from 19.7 ± 9.3 to 20.7 ± 6.3µV, P = 0.67). Finally, there were no significant correlations between TAV at admission and clinical parameters as age , LVEF and myocardial infarction extension, evaluated with the peak of serum creatine kinase concentration (Table 2).
| TAV Before CAG | ||
| ρ | P value | |
| Age | 0.11 | 0.33 |
| LVEF | -0.03 | 0.79 |
| ↑ CK* | 0.26 | 0.08 |
Abbreviations: TAV = T-wave amplitude variability; CAG = coronary angiography; LVEF = left ventricular ejection fraction; CK = creatine kinase. *Referred as percentage increase from normality according to sex and computed only in patients with an increase above normality limits (n = 45).
DiscussionTop
This study shows an increase in TAV in patients admitted for acute myocardial infarction without ST-segment elevation and treated with percutaneous myocardial revascularization. The increase of this index of ventricular repolarization dispersion was detectable in patients who underwent revascularization of the left anterior descending coronary artery, in comparison to patients subjected to reperfusion of circumflex or right coronary artery or who were not revascularized.
Ischemia and myocardial infarction are the most common substrates for the occurrence of malignant ventricular arrhythmias [24-26]. During ischemia several alterations of normal myocardial environment, particularly in beat-to-beat intracellular calcium homeostasis [27-30] and cell-to-cell coupling [27, 31, 32] can create an electrophysiological substrate prone to generate repolarization heterogeneity, conduction blocks and reentry, the latter considered as the major mechanism responsible for ischemia-induced arrhythmias [26]. Paradoxically, in addition to ischemia, reperfusion of the ischemic myocardium seems to be equally arrhythmogenic. In the clinical setting, this phenomenon is commonly referred as “reperfusion arrhythmias”, which frequently occur early after blood flow restoration and is considered an index of successful reperfusion [8, 25, 33-35].
Among the various available methods for detecting alteration of ventricular repolarization, mTWA, which detect a repeating ABABAB pattern in T-wave morphology and amplitude [8], has been considered the most sensitive and promising methodology to evaluate the risk for ventricular arrhythmic events [3, 4, 7, 36]. However, the spectral method, the most validated technique to detect mTWA, needs to increase patient’s heart rate to 100-110 beats for few minutes by a sub-maximal exercise [8, 37]. Such target is not always achievable, particularly in heart failure, recent acute myocardial infarction or optimal beta-blocking therapy. In addition, indeterminate results are obtained in almost 12-47% of published studies [8, 31, 37], thus making the interpretation of the mTWA test disputable.
In this context, T-wave amplitude variability was recently proposed as a new methodology for measuring repolarization variability on electrocardiographic recordings collected during resting conditions [14]. An increase in TAV was visible in MADIT II patients with appropriate ICD therapy [14] and in long-QT syndrome patients compared with normal subjects [17]. Higher TAV values were observed in Olympic athletes compared to healthy young adults and in subjects with a history of ventricular arrhythmias in absence of structural heart disease [19, 23]. Moreover, this methodology has proved to predict an increased arrhythmic risk in patients with dilated cardiomyopathy, in subjects undergoing cardiac resynchronization therapy and in Chagas disease [15, 16, 18, 19].
In the present study, TAV was tested in the particular electrophysiological environment represented by an ischemic myocardium, acutely subjected to blood flow restoration. We found an increase of TAV after myocardial revascularization following an acute subendocardial infarction. The increase was evident in subjects who underwent PTCA of LAD artery, a finding likely to be related to the extensive portion of myocardial muscle served by this vessel. Conversely, patients who were not revascularized did not show any significant change in TAV before and after angiography, as a likely result of both lack of effective mechanical revascularization and sudden restoration of coronary blood flow. The apparently paradoxical result of an increase in TAV after restoration of coronary blood flow can be considered as an indirect evidence of the so-called reperfusion injury: restoration of blood flow may lead to the development of reactive oxygen species and to the worsening in intracellular calcium overload [38], thus exacerbating ischemia-induced cellular injury, known to affect the repolarization process, either in its mechanical and electrophysiological characteristics [39]. This interpretation is supported by the results of a previous study, which reported a transient increase in mTWA during ballooning inflation and a subsequent decrease of this parameter 24hrs after the interventional procedure. In our study, we compared the admission recording with that performed within 24hrs from coronary revascularization, thus focusing on an earlier stage, in which abrupt blood flow restoration on peri-infarct zone generates a transient paradoxical effect [40]. Decrease in mTWA 24hrs after revascularization, as evidenced by the Batur et al. [41], would not be surprising or inconsistent with our results, being an expression of the progressive return to normal cellular metabolism of the ischemic zone, after successful restoration of blood flow. Interestingly, the same study reported more pronounced differences in mTWA after LAD angioplasty compared to other coronary vessels, similarly to what we observed [41]. Afterward, the Occluded Artery Trial – Electrophysiological Mechanism (OAT-EP) reported a decrease in TAV after coronary revascularization, but the after-procedure recording was obtained at 1 year from index event [42].
As to the values of TAV observed in the present study, it must be recalled that their averaged values did not differ notably from those measured in healthy subjects or in patients with minimal heart disease [17, 23]. In our opinion, this was due to the characteristics of our study population with normal QTc and, especially, preserved LVEF. Available literature established different cut-offs to identify patients at high risk for arrhythmic events, all characterized by TAV values ranging from 30 to 66µV [14-16, 18], likely to reflect the enrollment of patients with more severe cardiac conditions such as, for example, the MADIT II population or a previous history of sudden cardiac death [14-16, 18]. It may pertinent to recall that none of our patients presented sustained ventricular arrhythmias after coronary revascularization.
Finally, it is interesting to note that, also in our study population, the segments T4 and T5 of the repolarization phase, which correspond to the T wave, were the most significant and sensitive in detecting variations in repolarization process, thus confirming the capability of this methodology to detect changes in the final part of repolarization process [14-19, 23].
Limitations
Our study has several limitations: the small sample size is the principal one. This was partially due to the fact that, in order to avoid any delay in the appropriate treatment, we were unable to repeat another recording if an excessive noise made impossible the computation of TAV. This was the reason for which 20% of patients were excluded from data analysis. In addition, recordings were obtained at different time of the day but not during the night, thus limiting, but not excluding, the possible effects of circadian pattern of variations in autonomic control mechanisms [17]. The two subset of patients were not fully balanced: there was a relative greater number of diabetic patients in the revascularized group, but mean heart rate interval, QT and QTc interval, systolic arterial pressure and left ventricular ejection fraction were not different among groups, thus making unlikely that these factors might have affected our measurements [43].
ConclusionTop
Percutaneous myocardial reperfusion in NSTE-MI patients causes alterations in the ventricular repolarization that can be detected with this noninvasive electrocardiographic technique. The increase of TAV was evident in patients with LAD revascularization, as a likely result of the amount of myocardial tissue served by this vessel. TAV segments corresponding to the ascendant and descendent part of the T-wave appear to be the most appropriate to detect the reperfusion-induced repolarization changes. This approach, which deserves further clinical validation, seems therefore suitable to noninvasively detect transient alterations in the repolarization process.
Conflicts of interest
Authors declare no conflicts of interest.
ReferencesTop
[1]Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death. A scientific statement from the American Heart Association Council on clinical cardiology Committee on electrocardiography and arrhythmias and Council on epidemiology and prevention. J Am Coll Cardiol. 2008; 52(14):1179–1199.Article Pubmed
[2]Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J Am Coll Cardiol. 2009; 54(9):747–763.Article Pubmed
[3]Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006; 47(2):456–463.Article Pubmed
[4]Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004; 110(14):1885–1889.Article Pubmed
[5]Verrier RL, Nearing BD, La Rovere MT, Pinna GD, Mittleman MA, et al. Ambulatory electrocardiogram-based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol. 2003; 14(7):705–711.Article Pubmed
[6]Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004; 44(7):1481–1487.Article Pubmed
[7]Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009; 53(6):471–479.Article Pubmed
[8]Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, et al. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility - Consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol. 2011; 58(13):1309–24.Article Pubmed
[9]Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force and the European Society of Cardiology Committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol. 2006; 48(5):247–346.Article Pubmed
[10]Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RFE, Tritto M, et al. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. J Am Coll Cardiol. 2007; 50(19):1896–1904.Article Pubmed
[11]Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005; 46(1):75–82.Article Pubmed
[12]Hohnloser SH, Ikeda T, Cohen R. Evidence regarding clinical use of microvolt T-wave alternans. Heart Rhythm. 2009; 6(3 suppl):S36–S44.Article Pubmed
[13]Ikeda T, Yoshino H, Sugi K, Tanno K, Shimizu H, et al. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. J Am Coll Cardiol. 2006; 48(11):2268–2274.Article Pubmed
[14]Couderc JP, Zareba W, McNitt S, Maison-Blanche P, Moss AJ. Repolarization variability in the risk stratification of MADIT II patients. Europace. 2007; 9(9):717–723.Article Pubmed
[15]Žižek D, Cvijić M, Tasič J, Jan M, Frljak S, et al. Effect of cardiac resynchronization therapy on beat-to-beat T-wave amplitude variability. Europace. 2012; 14(11):1646–1652.Article Pubmed
[16]Ribeiro AL, Rocha MO, Terranova P, Cesarano M, Nunes MD, et al. T-wave amplitude variability and the risk of death in Chagas disease. J Cardiovasc Electrophysiol. 2011; 22(7):799–805.Article Pubmed
[17]Extramiana F, Tatar C, Maison-Blanche P, Denjoy I, Messali A, et al. Beat-to-beat T-wave amplitude variability in the long QT syndrome. Europace. 2010; 12(9):1302–1307.Article Pubmed
[18]Tasič J, Zupan I. T-wave variability as a risk stratifier in patients with dilated cardiomyopathy. PACE. 2009; 32:S155–S157.Article Pubmed
[19]Sobue Y, Watanabe E, Yamamoto M, Sano K, Harigaya H, et al. Beat-to-beat variability of T-wave amplitude for the risk assessment of ventricular tachyarrhythmia in patients without structural heart disease. Europace. 2011; 13(11):1612–1618.Article Pubmed
[20]Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012; 33(20):2551–25567.Article Pubmed
[21]Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevatio. Eur Heart J. 2011; 32(23):2999–3054.Article Pubmed
[22]Badilini F. The ISHNE holter standard output file format. Ann Noninvasive Electrocardiol. 1998; 3:263–266.
[23]Heinz L, Sax A, Robert F, Urhausen A, Balta O, et al. T-wave variability detects abnormalities in ventricular repolarization: a prospective study comparing healthy persons and olympic athletes. Ann Noninvasive Electrocardiol. 2009; 14:276–279.Article Pubmed
[24]Katritsis DG, Josephson ME. Sudden cardiac death and implantable cardioverter defibrillators: two modern epidemics Europace. 2012; 14(6):787–794.Article Pubmed
[25]Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012; 125(8):1043–1052.Article Pubmed
[26]Zipes DP, Wellens HJJ. Sudden cardiac death. Circulation. 1998; 98(21):2334–2351.Article Pubmed
[27]Bernus O, Zemlin CW, Zaritsky RM, Mironov SF, Pertsov AM. Alternating conduction in the ischaemic border zone as precursor of reentrant arrhythmias: a simulation study. Europace. 2005; 7 Suppl2:S93–104.Article Pubmed
[28]Merchant FM, Sayadi O, Moazzami K, Puppala D, Armoundas AA. T-wave alternans as an arrhythmic risk stratifier: state of the art. Curr Cardiol Rep. 2013; 15(9):391-398.Article Pubmed
[29]Hüser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, et al. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000; 524:795–806.Article Pubmed
[30]Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol. 2008; 294(1):H1–H10.Article Pubmed
[31]Armoundas AA, Tomaselli GF, Esperer HD. Pathophysiological basis and clinical application of T-wave alternans. J Am Coll Cardiol. 2002; 40(2):207–217.Article Pubmed
[32]Poelzing S, Akar FG, Baron E, Rosenbaum DS. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am J Physiol Heart Circ Physiol. 2004; 286(5):H2001–2009.Article Pubmed
[33]Six AJ, Louwerenburg JH, Kingma JH, De Medina EOR, van Hemel NM. Predictive value of ventricular arrhythmias for patency of the infarct-related coronary artery after thrombolytic therapy. Br Heart J. 1991; 66(2):143–146.Article Pubmed
[34]Goldberg S, Greenspon AJ, Urban PL, Muza B, Berger B, et al. Reperfusion arrhythmia: a marker of restoration of antegrade flow during intracoronary thrombolysis for acute myocardial infarction. Am Heart J. 1983; 105(1):26–32.Pubmed
[35]Wit AL, Janse MJ. Reperfusion arrhythmias and sudden cardiac death: a century of progress toward an understanding of the mechanism. Cir Res. 2001; 89(9):741-743.Article Pubmed
[36]Cutler MJ, Rosenbaum DS. Risk stratification for sudden cardiac death: is there a clinical role for T wave alternans Heart Rhythm. 2009; 6(8 Suppl):S56–61.Article Pubmed
[37]Bloomfield D, Hohnloser S, Cohen R. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol. 2002; 13(5):502–512.Article Pubmed
[38]Verma S, Fedak PW, Weisel RD, Butany J, Rao V, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002; 105(20):2332–2336.Article Pubmed
[39]Ferrero JM, Trenor B, Romero L. Multiscale computational analysis of the bioelectric consequences of myocardial ischaemia and infarction. Europace. 2014; 16(3):405-415.Article Pubmed
[40]Saeed M, Hetts S, Wilson M. Reperfusion injury components and manifestations determined by cardiovascular MR and MDCT imaging. World J Radiol. 2010; 2(1):1–14.Article Pubmed
[41]Batur MK, Oto A, Ider Z, Aksöyek S, Kabakci G, et al. T wave alternans can decrease after coronary revascularization. Angiology. 2000; 51:677–87.Pubmed
[42]Rashba EJ, Lamas GA, Couderc JP, Hollist SM, Dzavik V, et al. Electrophysiological effects of late percutaneous coronary intervention for infarct-related coronary artery occlusion: the Occluded Artery Trial-Electrophysiological Mechanisms (OAT-EP). Circulation. 2009; 119(6):779–787.Article Pubmed
[43]Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007; 115(3):387–397.Article Pubmed



