Journal of Clinical and Interventional Cardiology
An International Peer-Reviewed Open Access Journal
ISSN 2399-8202

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Clinical and Interventional Cardiology
Volume 1, Issue 2, October 2016, Pages 7–11
Original researchOpen Access
Long term follow-up post catheter intervention for critical pulmonary valve stenosis and atresia with intact ventricular septum: A 25-year single institutional experience utilizing various techniques
- 1 Pediatric Cardiology Division, Department of Pediatrics, Penn State Hershey Medical College, Hershey, Pennsylvania, USA
*Corresponding author: Howard Weber, M.D, FSCAI, Pediatric Cardiology, Department of Pediatrics, Penn State Hershey Medical Center, H085 500 University Drive, Hershey, Pennsylvania, USA. Tel.: 717 531 0000 ext. 8638; Fax: 717 531 2052; E-mail: hweber@hmc.psu.edu
Received 06 June 2016 Revised 20 August 2016 Accepted 15 September 2016 Published 22 September 2016
DOI: http://dx.doi.org/10.14312/2399-8202.2016-2
Copyright: © 2016 Nair A, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
Objective: Compare various catheter based interventions for critical pulmonary stenosis (PS) and pulmonary atresia with intact ventricular septum without right ventricular dependent coronary circulation (PA) regarding outcomes, predictors and survival. Results: Sixty two neonates underwent intervention at 4 days (weight: 3.3 kg). Fifty one had PS and 11 had PA with one procedural mortality (1.6%). Post intervention, 11 patients (18%) required a surgical BT shunt of which six eventually required univentricular palliation. Multivariate analysis predicted the initial tricuspid valve Z score was an independent predictor for BT shunt placement (mean -3.1; CI: -2.2 to -3.9 vs -1.9; CI: -1.3 to -2.5, p < 0.017). Initial pulmonary valve Z score was an independent predictor for both BT shunt placement (mean -1.6; CI: -2.1 to -1.1 vs -0.4; CI: -0.6 to -0.2, p < 0.001) and univentricular palliation (mean -1.4; CI: -2.2 to -0.67 vs -0.5; CI: -0.8 to -0.3, p = 0.029). Mean follow up is 10 years with a 93% survival. 25-year freedom from valve re-intervention was similar in patients with a biventricular repair requiring a BT shunt vs not. Four patients (7%) eventually required surgical pulmonary valve replacement (mean: 11 years; range 0.81- 19.82 years). Conclusion: A smaller tricuspid valve annulus diameter was an independent variable for BT shunt placement. A smaller pulmonary valve annulus was an independent predictor for BT shunt placement and subsequent univentricular palliation. 25-year survival is excellent with a low incidence of valve re-intervention or replacement in those patients with a biventricular circulation.
Keywords: critical pulmonary valve stenosis; pulmonary valve atresia; pulmonary valvuloplasty; laser perforation
IntroductionTop
Critical pulmonary stenosis (PS) and pulmonary valve atresia with intact ventricular septum and non-right ventricular dependent coronary circulation (PA) are rare congenital heart defects. They are characterized by systemic hypoxemia with spontaneous ductus arteriosus closure after birth and maintaining patency of the ductus arteriosus is essential for survival. Typical associated conditions are significant tricuspid insufficiency, variable hypoplasia of the right heart structures, right ventricular hypertrophy, right ventricular dysfunction and obligatory right to left shunting at the atrial level. Prior studies have demonstrated that a bipartite right ventricle (RV) and a tricuspid valve (TV) annulus Z score < -3 are associated with the need for supplemental blood flow to the pulmonary arteries via a surgical Blalock Taussig (BT shunt) and a trend toward subsequent univentricular palliation [1, 2] as the RV is unable to support systemic cardiac output. Univentricular palliation includes a second stage bidirectional Glenn anastomosis and finally an intra or extracardiac Fontan palliation procedure. Patients who do not require univentricular palliation achieve a biventricular circulation.
Following initiation of prostaglandin E1 (PGE1) to maintain ductal patency, management is directed at reestablishing patency of the right ventricular outflow tract (RVOT) although it is imperative to rule out RV dependent coronary circulation. Several percutaneous techniques with varied success have been described for perforation of the atretic pulmonary valve. Early experience utilized stiff guide wire perforation of the valve plate with or without arterial snare assistance due to extreme difficulty advancing even small diameter balloon dilation catheters across the valve. Valve perforation is now typically performed utilizing radiofrequency energy (RF) [3-5] and this method has been described extensively in the literature.
The first 28 patients of this study’s cohort were described with short term outcomes previously [6]. Since 2001, our preferred method has been to perforate or enlarge the critically stenotic pulmonary valve orifice utilizing a 0.9 mm diameter excimer laser catheter (Spectranetics Corporation, Fremont, CA) which has simplified the procedure [6].
We describe our 25-year institutional experience treating a large cohort of patients with PS or PA using these various percutaneous methods. Retrospectively, we aim to describe the echocardiographic and/or angiographic predictors of patients requiring a surgical BT shunt post percutaneous intervention and eventual univentricular palliation vs those achieving a biventricular circulation. Furthermore, we aim to describe the early and long term outcome of various percutaneous methods of pulmonary valve perforation in PS and PA.
Materials and methodsTop
Since 1989 to 2014, 62 patients presented to our center with PS or PA who were suitable for catheter based intervention based on transthoracic echocardiographic measurements. Patients who had severe RV hypoplasia (unipartite ventricle), severe PV hypoplasia (Z score > -3), severe TV hypoplasia (Z score > -4) or RV dependent coronary circulation were all referred for surgical intervention and are not included in our study.
The maximum pulmonary valve annulus diameter was measured from the transthoracic echocardiogram from the parasternal short axis view and was available in all patients. Forty-four patients had echocardiographic measurements and the remaining 18 were obtained via angiography at the time of cardiac catheterization. The TV annulus was measured from the apical four chamber view at end diastole and was available in 44 patients. The TV annulus was measured by the author retrospectively for the purpose of the study. Echocardiographic images of the 18 non-included patients were previously stored on videotapes which were no longer available for review. The 44 patients whom the measurements were available via digital archiving, were included in the analysis of outcome and the remaining 18 patients were excluded from the TV analysis.
All patients were then taken to the catheterization lab to assess for RV dependent coronary artery circulation prior to attempting to reestablish patency of the RVOT. No patients included in this study had angiographic evidence of RV dependent coronary artery circulation and therefore were considered candidates for catheter based therapy.
Statistics
The statistical analysis was done via SPSS software. Since the comparing variables were of different sample sizes, we used the Brown- Forsythe and Welch statistical analysis. Both analysis was found to have comparable results. Multivariate analysis using MANOVA was also used to predict factors that influenced if a patient would require a BT shunt or subsequent univentricular palliation after percutaneous intervention. Multivariate analysis was used to establish independent variables predictive of our outcome with sound statistical significance.
Z score is a numerical measurement of a value's relationship to the mean in a group of values. The Z score in our study was determined by available measurements from the Boston data.
Interventional techniques
Early experience (1990- 2000)
An initial RV angiogram was obtained. If trivial antegrade pulmonary blood flow was evident, a #4 French Judkins right coronary catheter was manipulated such that the tip was positioned immediately below the pulmonary valve annulus through which a floppy tipped (0.014 or 0.018 inch diameter) coronary guide wire was advanced. Once across the critically stenotic pulmonary valve orifice, the guide wire was positioned across the ductus arteriosus into the descending aorta. A gradational dilation approach, utilizing small diameter coronary artery balloons, followed by larger conventional balloon dilation catheters was used to relieve the valve obstruction. This technique could require up to 4 separate balloon dilation catheters in order to achieve a desired result. This technique was subsequently replaced by umbilical artery snare assistance which eliminated the need for a gradational approach. Once a guide wire was successfully advanced across the pulmonary valve and into the descending aorta via the ductus arteriosus, a 5 mm diameter gooseneck snare, via an umbilical or femoral artery catheter, was used to create a veno-arterial loop. This facilitated the placement of larger diameter dilation catheters across the valve, thereby shortening the procedure and fluoroscopy exposure [6]. If pulmonary valve atresia was evident, then stiff guide wire perforation of the valve was attempted utilizing the #4 French JR4 catheter as mentioned previously. If successful, then a floppy tipped coronary wire was advanced across the ductus arteriosus and into the descending aorta. The various methods described above for the pulmonary valvuloplasty were then utilized.
Later experience (2001- 2014)
If trivial antegrade pulmonary blood was evident via angiography, a #4 French Judkins right coronary catheter was manipulated such that the tip was positioned immediately below the pulmonary valve annulus. A floppy tipped 0.014 inch coronary guide wire was then advanced across the valve and into the descending aorta via the ductus arteriosus. If a low profile Mini-Tyshak (BBraun Corporation, Bethlehem, PA) balloon dilation catheter (balloon: annulus ratio of approximately 1.0-1.2) could not be advanced across the critically stenotic orifice, then laser enlargement of the critically stenotic orifice was performed. A 0.9 mm diameter excimer laser catheter was advanced over the existing trans pulmonary guide wire and up against the pulmonary valve plate following which energy was applied. The catheter would immediately advance across the valve and into the main pulmonary artery resulting in a 1mm diameter orifice through which the Mini-Tyshak balloon catheter could be easily advanced. The valve was then successfully dilated using a single balloon dilation catheter. If pulmonary valve atresia was evident, then a #6 French Zuma coronary guide catheter (Judkins right configuration) was manipulated such that the tip was positioned immediately below the atretic pulmonary valve which was confirmed via small contrast injections. The 0.9 mm diameter excimer laser catheter was then advanced up against the atretic pulmonary valve plate and once satisfactory positioned was confirmed, energy was applied resulting in immediate advancement of the laser catheter into the main pulmonary artery. A 0.014 inch floppy tipped coronary guide wire was then advanced through the lumen of the laser catheter and into the descending aorta via the ductus arteriosus. A low profile Mini-Tyshak balloon dilation catheter (balloon to annulus ratio of approximately 1.0 - 1.2) was then easily advanced and the valve successfully dilated (Figure 1 a-f).

ResultsTop
The mean age at intervention was 3.6 days (range 0- 69 days) and 61 patients were less than 7 days of age (Table 1). A single patient presented from outside the U.S at 69 days of age with PA, a restrictive patent ductus arteriosus and severe cyanosis. Mean weight was 3.3 kg (range: 1.8-5.4 kg). Fifty-one patients (82%) had PS and 11 (18%) had PA via angiography. Three patients had associated chromosomal abnormalities that were not considered lethal at the time of intervention and 60 patients (98%) were maintained on PGE1 for ductal dependent pulmonary blood flow pre intervention.
| Characteristics | N = 62 |
| Age at cath | 3.6 (0-69) days |
| Sex (male/female) | 32/30 |
| Weight | 3.3 (1.8-5.4) kg |
| Pulmonary stenosis/ Pulmonary atresia | 51/11 |
| PgE dependant | 60 |
| Chromosomal abnormality | 3 |
| Balloon: annulus ratio | 1.2 (1- 1.4) |
| Number of balloons useda | 1 (1-5) |
Note: aThe number of balloons used it in each different methods were statistically not significant.
Pulmonary valvuloplasty was successful in 61 patients (98%) with a single procedural mortality (1.6%) (Table 2). This occurred in a patient with PA during initial laser perforation of the valve. The infundibulum was inadvertently perforated without clinical consequences and the valve was then successfully laser perforated. Following valvuloplasty with an appropriate diameter balloon dilation catheter, an infundibular rupture occurred and cardiac tamponade ensued which could not be controlled.
| Parameter | Value |
| Procedure mortality1 | 1.6 % (N = 1) |
| Mortality secondary to other causes (Early) | 0.0% |
| Mortality secondary to other causes (Late)2 | 4.8 % (N = 3) |
| Clinical follow up (N = 57) | 117.4 (1-300) months |
| Re-intervention (excluding shunt patients) | 17.5% (N = 10) |
| Re-intervention (including shunt patients) | 31.0% (N = 18) |
| Repeat balloon valvuloplasty | 10.5% (N = 6) |
| Pulmonary valve replacement | 7.0% (N = 4) |
Note: 1Mortality secondary to RVOT perforation and cardiac tamponade; 2Mortality secondary to dilated cardiomyopathy, unknown cause and secondary to Meinke’s syndrome.
Nineteen patients were suspected to have pulmonary atresia via transthoracic echocardiography. Eleven patients were confirmed at angiography to have pulmonary atresia whereas eight patients had trivial antegrade pulmonary blood flow. Specificity for transthoracic echocardiography to detect PA was 84% (CI- 71%- 93%) with a sensitivity of 100% (CI- 72%-100%). The positive predictive value for echocardiography to detect PA is 58% (CI- 34-80%). The negative predictive value is 100% (92%-100%). The positive likelihood ratio is 6.25 and negative likelihood ratio was zero.
At angiography, PA patients had an increased incidence of a bipartite RV (82%) compared to 31% of PS patients (p = 0.003). Three of 11 PA patients and 3 out of 51 PS patients eventually underwent univentricular palliation and all had bipartite ventricles (p = 0.32). The mean balloon: annulus ratio was 1.2 (range: 1-1.4). The fluoroscopy exposure time in patients with PA undergoing laser perforation was not statistically different when compared to the remaining PA patient group who underwent other percutaneous methods. Twenty five patients (40 %) were maintained on PGE1 post catheter intervention (mean 10 days; range 1-34 days). The difference in PGE1 duration between those requiring a shunt and not requiring a shunt was statistically significant (15 days; CI: 16.6-23.4 vs 5.9 days; CI: 2-9.8 days, p < 0.0015).
On analysis of the 44 patients with an available TV Z score, the initial TV Z score was an independent predictor for BT shunt placement but not univentricular palliation (mean: -3.1; CI: -2.2 to -3.9 vs -1.9; CI: -1.3 to -2.5, p = 0.017). A reduced pulmonary valve Z score was found to be an independent predictor for both BT shunt placement (mean: -1.6; CI: -2.1 to -1.1 vs -0.4; CI: -0.6 to -0.2, p < 0.001) and univentricular palliation (mean: -1.4; CI: -2.2 to -0.67 vs -0.5; CI: -0.8 to -0.3, p = 0.029).
Mean follow up duration for the 57 surviving patients (93%) was 9.8 years (range 0.08-25 years). Three patients expired during follow-up secondary to LV dilated cardiomyopathy (n = 1), complications of Meinke’s disease (n = 1) and unknown etiology in one. Five patients (8%) required repeat balloon valvuloplasty at a mean duration of 49 days from initial balloon valvuloplasty (range: 4 - 944 days). Four patients (7%) required late surgical pulmonary valve replacement a mean of 11 years (range 0.81- 19.82 years) post neonatal intervention. Twenty-five-year freedom from catheter or surgical re-intervention in biventricular repair patients was similar between those requiring vs not requiring a BT shunt (Figure 2).
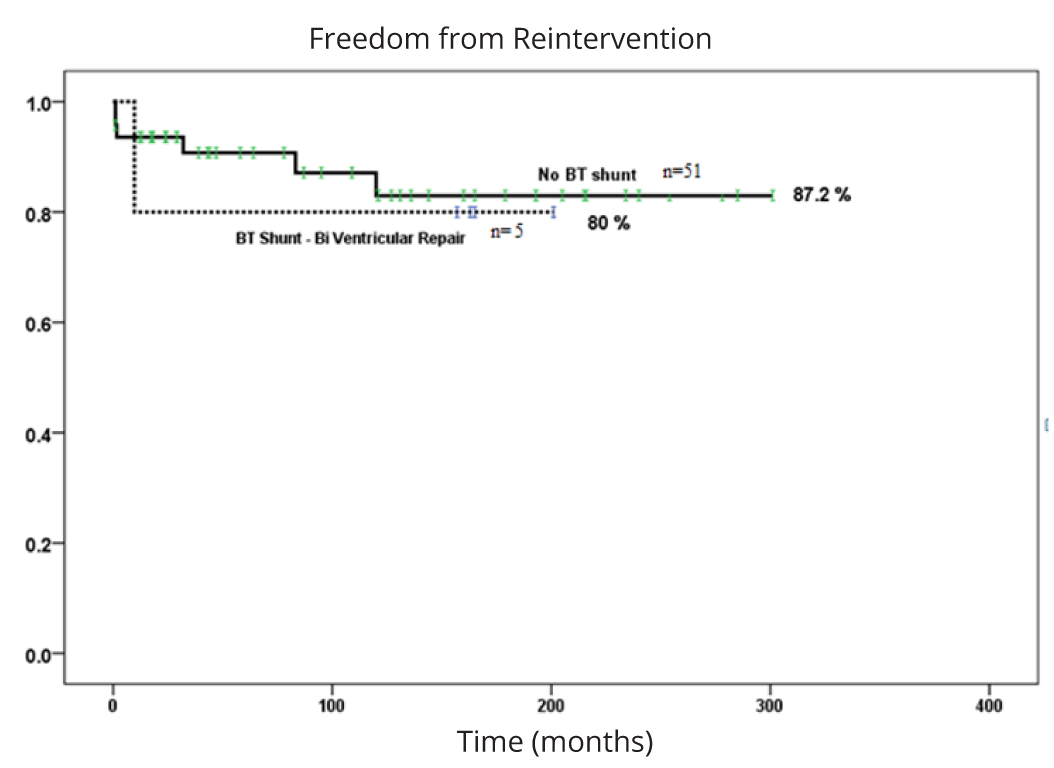
Abbreviations: PS: critical pulmonary stenosis, PA: pulmonary atresia with intact ventricular septum with nondependent coronary circulation, RV: right ventricle, RF: radiofrequency energy, TV: tricuspid valve, RVOT: right ventricular outflow tract, PgE: prostaglandin E1 infusion.
DiscussionTop
We have reported a large single center >25-year experience for catheter based intervention in neonates with PS or PA. PA and PS were combined as single cohort since these patients share comparable anatomical and physiological attributes and initial management is identical. In patients with severe right heart hypoplasia, the decision to pursue surgical palliation is easier although interventional techniques are less invasive but not in the patient’s best interest. The techniques utilized in alleviating RVOT obstruction in these patients have undergone dramatic changes over the last 3 decades. Early experience utilized stiff guide wire perforation of the atretic pulmonary valve with umbilical artery snare assistance which has transitioned to RF ablation and our more recent experience utilizing the excimer laser catheter. This evolution has led to a simplification of the procedure. Although we were unable to establish statistical significance for a reduced fluoroscopy exposure in the PA patients who underwent laser intervention (N = 6), this is likely secondary to the small sample sizes (N = 11). In addition, a new method typically has a learning curve and there may be increased or similar fluoroscopic exposure times compared to conventional methods until the technique is mastered and the user is more confident. This trend was observed within our PA group who underwent the laser method.
We also found that transthoracic echocardiography is an excellent, diagnostic tool for suspected pulmonary valve atresia, although it may over diagnose it and confirmation via angiography remains the gold standard.
We also demonstrated that the initial transthoracic echo TV Z score was an independent factor for subsequent BT shunt placement but not eventual univentricular palliation. As the measurements were retrospectively obtained, we can speculate that this might likely translate into an independent predictor for subsequent BT shunt placement. Clinically, this could lead to a shorter duration on PGE1 post procedure with earlier surgical BT shunt placement or ductal stenting. We found that a reduced pulmonary valve Z score was an independent predictor for both BT shunt placement and univentricular palliation despite successful decompression of the right ventricle. RV morphology did not independently correlate to these 2 outcomes. This is contrary to existing studies which have concluded that the TV Z score and bipartite RV morphology was predictive for univentricular palliation and the pulmonary valve Z score was not a dependent variable of ventricular outcome [1, 2].
Strengths of the study
We have described a large number of PA and PS patients with long term follow up. Comparison of different methods of a single operator reduces inter-user variability of results and outcomes.
Weakness of the study
This is a retrospective, single center study. Selection bias may occur in a single center study. Furthermore, the patients chosen for the percutaneous methods were pre-selected by known echocardiographic criteria by our institutional department consensus. The study involved the experience of only 1 interventionalist and experience and outcomes may vary with different operators. We believe a multicenter randomized controlled trial with multivariate analysis may help to further confirm the findings of this study. However, the ability to accomplish this would be limited by the nature of the disease and the ethics surrounding it. Echocardiographic measurements were not validated by inter or intra- observer differences.
Future direction
We continue to use the echocardiographic predictors we have described in conjunction with the laser perforation method in future patients with PS or PA in order to determine whether there is a need for surgical BT shunt placement or ductal stenting.
ConclusionTop
Smaller TV annulus Z score was an independent variable predictive for BT shunt placement. Smaller pulmonary valve annulus Z score was an independent predictor for BT shunt placement and subsequent univentricular palliation. Laser perforation in patients with PS or PA is an effective method of relieving the RVOT obstruction and with increasing experience should lead to reduced fluoroscopy exposure and complications. Overall 25-year survival for the entire cohort is excellent with a low incidence of catheter based re-intervention and surgical pulmonary valve replacement.
Conflicts of interest
Authors declare no conflicts of interest.
ReferencesTop
[1]Schwartz MC, Glatz AC, Dori Y, Rome JJ, Gillespie MJ. Outcomes and predictors of reintervention in patients with pulmonary atresia and intact ventricular septum treated with radiofrequency perforation and balloon pulmonary valvuloplasty. Pediatr Cardiol. 2014; 35(1):22–29.Article Pubmed
[2]Marasini M, Gorrieri PF, Tuo G, Zannini L, Guido P, et al. Long-term results of catheter-based treatment of pulmonary atresia and intact ventricular septum. Heart. 2009; 95(18):1520–1524.Article Pubmed
[3]Walsh MA, Lee KJ, Chaturvedi R, Arsdell GSV, Benson LN. Radiofrequency perforation of the right ventricular outflow tract as a palliative strategy for pulmonary atresia with ventricular septal defect. Catheter Cardiovasc Interv. 2007; 69(7):1015–1020.Article Pubmed
[4]Rosenthal E, Qureshi SA, Chan KC, Martin RP, Skehan DJ, et al. Radiofrequency-assisted balloon dilatation in patients with pulmonary valve atresia and an intact ventricular septum. Br Heart J. 1993; 69(4):347–351.Article Pubmed
[5]Alwi M, Choo KK, Radzi NAM, Samion H, Pau KK, et al. Concomitant stenting of the patent ductus arteriosus and radiofrequency valvotomy in pulmonary atresia with intact ventricular septum and intermediate right ventricle: Early in-hospital and medium-term outcomes. J Thorac Cardiovasc Surg. 2011; 141(6):1355–1361.Article Pubmed
[6]Weber HS. Initial and late results after catheter intervention for neonatal critical pulmonary valve stenosis and atresia with intact ventricular septum: A technique in continual evolution. Catheter Cardiovasc Interv. 2002; 56(3):394–399.Article Pubmed



