Journal of Clinical and Interventional Cardiology
An International Peer-Reviewed Open Access Journal
ISSN 2399-8202

- Download PDF
- |
- Download Citation
- |
- Email a Colleague
- |
- Share:
-
- Tweet
-

Journal of Clinical and Interventional Cardiology
Volume 1, Issue 1, May 2017, Pages 1–4
Case reportOpen Access
First application of ultrasound-assisted thrombolysis for acute, submassive pulmonary embolism in a pregnant patient
-
Rohan Kalathiya1,
Brian D Conway1,
John A Blair1,
Jonathan Paul1,
Atman P Shah1,
Mahmoud Ismail1 and
Sandeep Nathan1,*

- 1 Section of Cardiology, Department of Medicine, University of Chicago Medicine, Chicago, Illinois, USA
*Corresponding author: Sandeep Nathan, MD, MSc, Section of Cardiology, The University of Chicago Medicine, 5841 South Maryland Avenue, MC 5076, Chicago, Illinois 60637, USA. Tel.: (773) 702-2697; Fax: (773) 702-0241; E-mail: snathan@medicine.bsd.uchicago.edu
Received 17 March 2017 Revised 28 April 2017 Accepted 03 May 2017 Published 10 May 2017
DOI: http://dx.doi.org/10.14312/2399-8202.2017-1
Copyright: © 2017 Kalathiya R, et al. Published by NobleResearch Publishers. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
AbstractTop
We describe the first reported case in the peer-reviewed literature, in which ultrasound-assisted thrombolysis (USAT) for high risk, submassive pulmonary embolism (PE) was successfully utilized in a first trimester, pregnant patient (12 weeks gestation), wherein she successfully carried twins to term and underwent uncomplicated cesarean section. By reducing the required dose of fibrinolytic, this report demonstrates the use of USAT as a potentially safe and effective treatment modality for reducing clot burden and rapidly reversing right ventricular dysfunction in this difficult clinical scenario.
Keywords: EKOS; ultrasound-assisted thrombolysis; pregnancy; pulmonary embolism
IntroductionTop
The incidence of pulmonary embolism (PE) during pregnancy reportedly ranges from 0.76 to 1.72 cases per 1000 pregnancies, occurs four times more frequently than in a comparable non-pregnant population and is sustained in an equal distribution over all three trimesters. Pulmonary embolism thus remains one of the leading causes of maternal death in the developed world [1]. The true incidence of submassive and massive PE, however, is likely obscured, as it is in the general population, by the protean manifestations of the syndrome leading to misclassification bias along with its high pre-hospital case-fatality rate. This is especially true with massive PE when aggressive treatment is not promptly instituted.
The treatment of high risk PE has evolved over the past few decades from the administration of bolus-dose, systemic thrombolysis to more localized, catheter directed approaches. Catheter-directed treatments, however, have traditionally required the thrombolytic agent to permeate the clot burden in a relatively slow diffusion-dependent fashion, often necessitating multiple rounds of administration in conjunction with fluoroscopically-guided catheter repositioning and longer treatment duration [2, 3]. The concept of ultrasound-assisted thrombolysis was first introduced by Kudo in 1989, as an enhancement to catheter-directed thrombolysis (CDT), aimed at improving the contact of the fibrionolytic agent with plasminogen receptor sites in the thrombus to allow for decreased dose and treatment time [4]. In this report, we describe the first application of a commercially-available USAT system in an unstable pregnant female with an acute, submassive pulmonary embolism and severe right ventricular dysfunction, a scenario where either further hemodynamic compromise or major bleeding could be catastrophic to the mother or twin fetuses.
Case reportTop
A 23-year-old obese woman with a history of hypertension, two previous pregnancies, currently pregnant with twins (12-week gestation) and recently-diagnosed with extensive, bilateral lower extremity deep vein thrombosis (DVT) presented to the Emergency Department (ED) with sudden onset chest pain, shortness of breath and presyncope symptoms. She had presented to the ED two weeks before the index hospitalization with lower extremity swelling and was diagnosed with totally occlusive acute DVTs of the right popliteal, posterior tibial, and peroneal veins. She was discharged on weight-adjusted, low-molecular-weight heparin (enoxaparin 150 mg SC BID).
On presentation, heart rate was 115 beats per minute, blood pressure 110/60 mmHg (which was significantly reduced from her prior outpatient hypertensive baseline), respiratory rate 26 breaths per minute, and pulse oximetry 89-90% on room air. On examination, she was in obvious respiratory distress with a rapid regular heart rate with normal first and second heart sounds and clear lung fields on auscultation, gravid abdomen and trace bilateral lower extremity edema. Laboratory markers were notable for an anion gap of 20 mmol/l (reference 5-15 mmol/l), N-terminal pro-BNP 154 pg/ml (reference < 125 pg/ml), troponin T 0.11 ng/ml (reference < 0.03 ng/ml). She had missed the four prior doses of low-molecular-weight heparin after not refilling her prescription. Bedside echocardiogram revealed markedly dilated right ventricle with McConnell’s sign, moderate to severe hypokinesis and a flattened interventricular septum (Figure 1). Computed tomography was deferred due to her pregnant status.
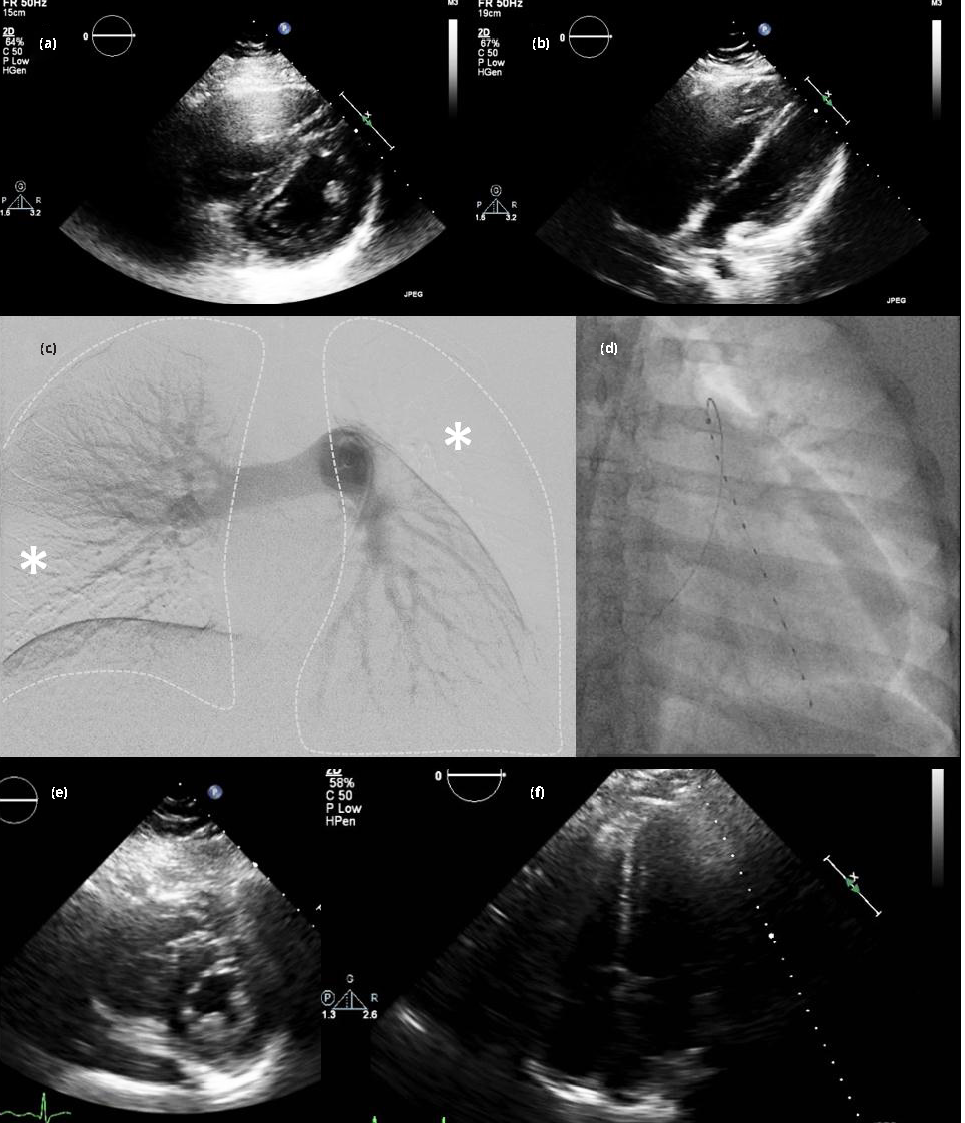
After extensive discussions with the maternal-fetal medicine service and interventional cardiology, she was brought to the cardiac catheterization laboratory for pulmonary artery catheterization and angiography for diagnosis and treatment of likely submassive PE. Given the high likelihood of PE, the potential risks to the patient and the developing fetuses conferred by CDT versus USAT versus medical management were explained in detail. The patients abdomen was shielded using lead aprons, and the right common femoral vein was accessed using ultrasound-guidance and an 18-gauge needle with a 7-French introducer sheath was advanced into the vessel. A 7-French balloon-tipped catheter was next advanced into the pulmonary artery (PA). The initial right atrial pressure was 13 mmHg, PA pressure 45/25/32 mean mmHg, PA saturation 54% and cardiac index 2.72 L/min/m2 using the Fick equation. Pulmonary angiography revealed multiple bilateral occlusive and sub-occlusive filling defects. Notable on the still image (Figure 1) there was complete occlusion of the left ascending pulmonary branch and its tributaries as well as numerous occlusions in the segmental vessels derived from the descending branches of the right and left PAs. The left PA was targeted for initial therapy given the greater thrombus burden on that side demonstrated by subsequent selective injections, taken together with the goal of minimizing total fibrinolytic dose.
A 300 cm, 0.035” angled Glidewire Advantage (Terumo Interventional Systems; Somerset, NJ, USA) was placed through the PA catheter, past the thrombus and into an occluded branch of left descending PA branch. The PA catheter was then exchanged for the EKOS ultrasound infusion catheter system (EKOS Corporation; Bothell, WA; Figure 1). A 2 mg bolus of alteplase was administered through the catheter followed by infusion of 0.5 mg/h for 24 h (total t-PA dose = 14 mg). Repeat echocardiography performed at 48 h revealed complete normalization of right ventricular size and function (Figure 1) and thus the decision was made to defer further catheter repositioning and additional fibrinolysis. A retrievable inferior vena cava filter was placed given the patient’s recent noncompliance and significant residual proximal thrombus burden in the femoral veins. She was discharged on low-molecular-weight heparin and underwent planned cesarean section due to breech presentation at 37 weeks, resulting in uncomplicated delivery of a healthy girl at 2.55 kg and boy at 2.76 kg. Her post-operative course was complicated by anemia requiring transfusion of two units packed red blood cells. She and the new members of her family were discharged after four days, and she was transitioned to warfarin therapy with a low-molecular-weight heparin bridge.
DiscussionTop
In the United States, the incidence of thrombotic PE (as differentiated from amniotic fluid emboli) during pregnancy is high and is listed by the US Centers for Disease Control and Prevention as the sixth leading cause of maternal death and responsible for roughly 9.2% of maternal mortality [5]. It is imperative that rapid, accurate diagnosis is made in order to expedite treatment and minimize long-term sequelae. The diagnosis of PE in pregnancy is difficult owing to the wide range of symptoms patients present with and concerns about radiation exposure to the fetus from conventional CT imaging. Distinguishing pregnancy-related symptoms from PE remains challenging since the most common signs and symptoms of PE (dyspnea, tachycardia and tachypnea) are also associated with a normal pregnancy. There is a paucity of literature describing the clinical diagnostic criteria for PE specific to pregnant patients [6]. However, one study looking at 38 pregnant patients with confirmed PE found the top four presenting features included dyspnea (62%), pleuritic chest pain (55%), cough (24%) and sweating (18%) [7]. Laboratory studies such as arterial blood gas, D-dimer and echocardiography are often performed but are neither sensitive nor specific in diagnosing a suspected PE. The diagnosis is therefore based on pretest probability for PE and balancing the diagnostic accuracy of confirmatory testing with the risk of exposure to the fetus. In our case, recent diagnosis of DVT followed by discontinuation of anticoagulation and subsequent acute dyspnea, elevated biomarkers and right ventricular dysfunction prompted expedited diagnosis using invasive pulmonary angiography.
The treatment for PE in pregnancy is also difficult owing to the risk of maternal bleeding and risks to the fetus with anticoagulation and fibrinolytic therapy. Warfarin is contraindicated, especially in the first trimester, given its ability to cross the placenta and cause both fetal bleeding and teratogenicity. In the setting of systemic thromboembolic disease, the use of low-molecular-weight heparin, such as enoxaparin, is generally preferred and should be continued through pregnancy. The placement of inferior vena cava filters can also minimize the risk of PE in pregnant patients at high risk for PE following the diagnosis of DVT and are unable or unwilling to comply with anticoagulation. Consideration for fibrinolytic therapy for PE in pregnant patients should be tailored to each patient based on the risk for hemodynamic deterioration. While the use of systemic thrombolytics in pregnant patients has been shown to improve morbidity and mortality following massive PE (large bilateral PE with hypotension/shock/cardiac arrest), this therapy carries a substantial risk of preterm labor, spontaneous abortion and placental abruption [8, 9]. Fibrinolytic therapy for pregnant patients with submassive PE (large bilateral PE without hypotension) is not well-established, however, a systematic review of case series and case reports consisting of 172 pregnant women treated with systemic thrombolytics found maternal mortality to be 1%, fetal loss 6% and the incidence of maternal hemorrhagic complications 8% [10, 11].
The use of CDT may mitigate the risk to the mother and fetus by administering the fibrinolytic medication locally, thereby reducing the overall administered dose or at least the dose per unit time [12]. USAT has emerged as a therapy to effectively administer low-dose fibrinolytic directly into the clot through an infusion catheter while ultrasound mechanically disrupts the fibrin and allows for acoustic streaming of the fibrinolytic into the clot [13]. The ULTIMA study randomized 59 non-pregnant patients with submassive PE, right ventricular dysfunction and elevated troponin to USAT with 10 to 20 mg of recombinant tissue plasminogen activator (t-PA) infused over 15 h plus unfractionated heparin versus unfractionated heparin alone. There was a significant improvement in RV dysfunction in the USAT plus heparin group compared to heparin alone, with a 10% risk of minor bleeding compared to 3%, and no major bleeds [14]. The larger, non-randomized SEATTLE II study demonstrated decreases in RV dilation, reduction in pulmonary hypertension, decreased anatomic thrombus burden and 10% rate of major bleeding in 150 patients with acute massive and submassive PE [10]. Subjects underwent infusion with 24 mg of t-PA through either a single catheter over 24 h or two catheters over 12 h. Success was demonstrated by reversal of the RV/LV ratio within 48 h of treatment cessation without any cases of intracranial bleeding and only one GUSTO major bleeding event.
Guidelines for the management of pulmonary embolism have been issued by a number of medical organizations including the American Academy of Family Physicians (AAFP)/ American College of Physicians (ACP), American College of Physicians (ACP), American College of Emergency Physicians (ACEP), American College of Radiology (ACR), American College of Chest Physicians, American Heart Association (AHA), and the American College of Obstetricians and Gynecologists (ACOG). A recent state of the art treatment update summarizing these recommendations was published by Konstantinides et al., with specific detail of the ULTIMA and SEATTLE II data and an accompanying treatment algorithm recommending primary reperfusion therapies in addition to anticoagulant therapy for high risk PE [15]. Furthermore, the authors of the review state that catheter-directed techniques should be considered for patients with hemodynamic decompensation and high bleeding risk for whom systemic fibrinolysis may not have a favorable risk-benefit ratio. The patient described in this report fulfilled high risk criteria based on her progressive cardiopulmonary compromise, relative hypotension and acute RV dysfunction by echocardiography and both patient and fetuses were at risk for potentially catastrophic bleeding complications with traditional doses of thrombolytics.
Thus, thrombolytic administration for acute massive or high risk submassive PE is slowly becoming an accepted adjunct to anticoagulation in certain scenarios. Until recently, thrombolytics were most often administered either as a systemic bolus or in a catheter directed fashion into the clot burden. Both CDT and USAT necessitate invasive pulmonary angiography and targeted catheter placement for thrombolytic administration, and therefore requires angiography facilities and equipment as well as local technical expertise. In the case of USAT however, the dose of thrombolytic agent is typically reduced as compared with other delivery strategies and slowly infused over a 12-24 h period with the aid of ultrasound to improve drug penetration into the clot. Both ULTIMA and SEATTLE II trials demonstrated safety and efficacy of USAT, with ULTIMA showing superiority to heparin anticoagulation alone with no excess in major bleeding complications. Thus, USAT using a reduced thrombolytic dose offers significant potential benefits to patients deemed to be high risk from the standpoint of the index pulmonary embolism, but also at a high risk for bleeding complications such as the pregnant patient described. It remains to be determined if clinical outcomes comparable to USAT are also achievable with (non-ultrasound-assisted) catheter-directed fibrinolytics at a reduced dose.
ConclusionTop
To our knowledge, this is the first reported case in the peer-reviewed medical literature highlighting the use of ultrasound-assisted thrombolysis in a pregnant patient, early in her pregnancy, with submassive PE and evolving high risk features. In this case, the morbidity and mortality from the PE, and the possibility of fetal loss from a bleed, were avoided by administering low-dose, localized, ultrasound-assisted fibrinolysis directly into the thrombus with rapid and complete reversal of severe right ventricular dysfunction. Electing this approach did not result in bleeding complications during or shortly after the index treatment for her pulmonary embolism, nor did it complicate the pregnancy, which resulted in the successful delivery of twins by planned cesarean section at 37 weeks.
Conflicts of interest
Dr. Paul has served as a consultant to Penumbra, Inc. The remaining authors report no relevant financial relationships or conflicts of interest.
ReferencesTop
[1]Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008; 359(19):2025–2033.Article Pubmed
[2]Sag S, Nas OF, Kaderli AA, Ozdemir B, Baran I, et al. Catheter-directed ultrasound-accelerated thrombolysis may be life-saving in patients with massive pulmonary embolism after failed systemic thrombolysis. J Thromb Thrombolysis. 2016; 42(3):322–328.Article Pubmed
[3]Francis CW. Ultrasound-enhanced Thrombolysis. Echocardiography. 2001; 18(3):239–246.Article Pubmed
[4]Kudo S. Thrombolysis with ultrasound effect. Tokyo Jikeikai Med J. 1989; 104:1005–1012.
[5]Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. United States. 2017.Article
[6]Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006; 354(22):2317–2327.Article Pubmed
[7]Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: The SEATTLE II study. JACC Cardiovasc Interv. 2015; 8(10):1382–1392.Article Pubmed
[8]Leonhardt G, Gaul C, Nietsch HH, Buerke M, Schleussner E. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis. 2006; 21(3):271–276.Article Pubmed
[9]Fasullo S, Maringhini G, Terrazzino G, Ganci F, Paterna S, et al. Thrombolysis for massive pulmonary embolism in pregnancy: A case report. Int J Emerg Med. 2011; 4:69.Article Pubmed
[10]Turrentine MA, Braems G, Ramirez MM. Use of thrombolytics for the treatment of thromboembolic disease during pregnancy. Obstet Gynecol Surv. 1995; 50(7):534–541.Pubmed
[11]Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation. 2011; 123(16):1788–1830.Article Pubmed
[12]Kuo WT, Banerjee A, Kim PS, Demarco FJ Jr, Levy JR, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest. 2015; 148(3):667–673.Article Pubmed
[13]Owens CA. Ultrasound enhanced thrombolysis: EKOS endowave Infusion Catheter System. Semin Intervent Radiol. 2008; 25(1):37–41.Pubmed
[14]Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014; 129(4):479–486.Article Pubmed
[15]Konstantinides SV, Barco S, Lankeit M, Meyer G. Management of pulmonary embolism: An update. J Am Coll Cardiol. 2016; 67(8):976–990.Article Pubmed


